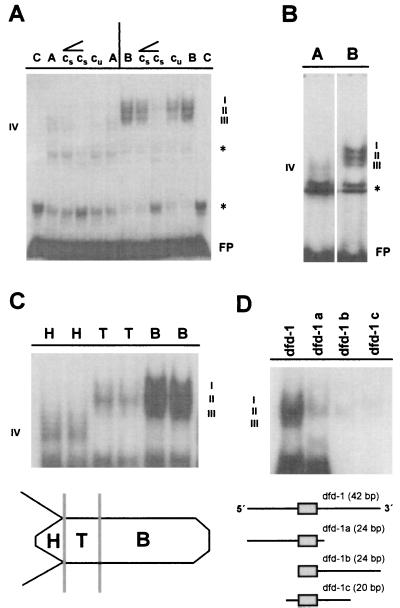
Figure 4.
DNA–protein interactions at the dfd-1 sequence element of the ks1 promoter. (A) Mobility shift experiment showing differential binding of nuclear protein from apical and basal tissue. (B) Higher resolution of DNA–protein interactions at the dfd-1 sequence element demonstrating formation of three complexes with nuclear proteins from basal tissue. (C) Gradient of nuclear proteins binding to the dfd-1 element along the body axis with sharp decrease of DNA–protein interactions in TFZ and head tissue. (D) Upper, DNA–protein interactions at subfragments dfd-1 a, dfd-1 b, and dfd-1 c of the dfd-1 site. Lower, Schematic representation of Dfd core sequence. C, control (without protein); A, nuclear extract from apical tissue; B, nuclear extract from basal tissue; H, nuclear extract from head tissue; T, nuclear extract from TFZ; cu, addition of unspecific competitor; cs, addition of specific competitor; ∠, rising concentrations of unspecific or specific competitior (10- or 100-fold molar excess); FP, free probe; ∗, binding of general nuclear protein(s) to oligonucleotide; I–IV, dfd-1–protein complexes. Sequence of the dfd-1 oligonucleotide is 5′-TTTGATTTGCGTATTAATTACAATTTAATAGATTTGAT-3′ (Dfd core sequence in italics).