Abstract
Saliva contains components of both the mucosal and systemic immune systems. Variable flow rates, immunoglobulin proteases, and variation in collection and storage methods all introduce differences in the estimated concentrations of antibodies. We evaluated the effect of four collection methods and three storage protocols on the concentrations of immunoglobulin A (IgA) antibodies to pneumococcal capsular antigens 1, 5, 6B, and 14 and to pneumococcal surface adhesin A (PsaA) in saliva. Specimens were collected from 30 healthy Kenyan adults by collecting drool, by pipette suction, and with two commercial kits, OraSure and Oracol. Aliquots from each specimen were snap-frozen with glycerol in liquid nitrogen or stored for 4 to 8 h at +4°C either with or without the addition of protease enzyme inhibitors prior to storage at −70°C. Anticapsular IgA concentrations were not significantly different with different collection methods, but snap-freezing the specimens in liquid nitrogen led to concentrations 41 to 47% higher than those of specimens stored by the other methods (P < 0.0005).
Oral fluid (OF) is an attractive specimen with which to assay specific aspects of the immune system. OF can be considered to represent the body's first defense against oropharyngeal pathogens. OF contains elements specific to mucosal immunity (e.g., immunoglobulin A [IgA] with a secretory component) but gives a simultaneous view of serum immune components, including IgG. The most useful feature of OF is that it can be obtained noninvasively.
OF has two significant disadvantages in assays for studies of immunity to bacteria. First, it contains bacterial protease enzymes which can degrade immunoglobulins, for example, IgA1 (9). This enzyme activity can be inhibited by freezing with glycerol or, theoretically, by antiprotease enzymes, although the assay of some antibodies is reported to be unaffected by storage at +4°C, even for several days (14). Second, immunoglobulin concentrations in OF are much lower than in serum and may be subject to diurnal and monthly variation (2). At low concentrations the relative importance of methodological differences due to variation in specimen collection and sample storage is much greater, yet there is little previous validation of OF sampling methods for assays of bacterial antibodies. Indeed, this is the first study to compare the effects of different collection and storage methods on antipneumococcal antibody concentrations in saliva.
The fluid circulating in the oral cavity consists of a mixture of saliva secreted by the salivary glands, gingival crevicular fluid, mucosal products, bacteria, viruses, hormones, antibodies, and traces of food (3, 6). Saliva can be collected by cannulation of the salivary ducts, but OF has the dual advantages of being easier to collect and more representative of the oral milieu.
Several studies suggest that salivary antibodies mediate immunity against Streptococcus pneumoniae and Haemophilus influenzae type b (Hib) carriage and, therefore, possibly against local and invasive disease. Anti-S. pneumoniae and anti-Hib IgA and IgG have been shown to reduce the carriage of S. pneumoniae and Hib in an infant rat model of nasopharyngeal colonization (8, 11). In humans, anti-S. pneumoniae and anti-Hib antibodies can facilitate the clearance of specific bacteria from middle ear fluid (19) and vaccination with S. pneumoniae and Hib conjugate vaccines induces the development of local antibodies (4, 7, 10, 15, 16, 17) and reduces colonization with the vaccine type of S. pneumoniae and Hib (1, 5, 12, 13).
In addition to containing IgA, OF contains IgG transudated from blood vessels into the oral cavity, and OF may be used simultaneously to evaluate local and systemic immunity both in disease and in response to vaccination (4, 10, 15, 16, 17, 18). A future study will examine the correlation of susceptibility to invasive pneumococcal disease with immune variables in serum and saliva. The present study evaluates the optimal conditions for the collection and storage of OF samples to maximize the yield of antipneumococcal antibodies, particularly anti-capsular polysaccharide (anti-[CPS]) IgA.
MATERIALS AND METHODS
Population.
Study subjects were 30 healthy Kenyan adult volunteers among the staff of the Wellcome Trust/Kenya Medical Research Institute, Kilifi, Kenya. They were asked not to eat or drink for 2 h before the study. OF specimens were collected from every subject by each of four different methods, conducted in random order 15 min apart.
Sample collection.
The following specimen collection methods were used. (i) For an unstimulated specimen, the subject was asked to drool into a clean 50-ml container. (ii) For a Pastette sample, the investigator sucked the OF from under the tongue and the paragingival gutter of the subject with a disposable plastic pipette. (iii) For an OraSure (Epitope, Beaverton, Oreg.) sample, a cotton pad on a short plastic stick was placed between the gums and the cheek and left there for 2 min. After removal from the mouth, the stick was broken off and the pad was placed in a storage container with approximately 800 μl of proprietary buffer. (iv) For an Oracol (Malvern Medical Developments Ltd., Worcester, United Kingdom) sample, a cylindrical plastic sponge mounted on a short wooden stick was used by the subject to brush his or her teeth, tongue, and gums for 60 s. The sponge was then placed into an Oracol tube with 1 ml of buffer containing 10% fetal calf serum (FCS) in 0.17 M phosphate-buffered saline (PBS) (DulbeccoA; Oxoid, Basingstoke, Hampshire, United Kingdom) (pH 7.3) with 10 μg of CPS (Statens Serum Institut, Copenhagen, Denmark) per ml. The OraSure and Oracol samples were centrifuged for 5 and 10 min, respectively, at 3,000 rpm before the pad or sponge was removed.
Sample storage.
Each specimen was divided into three equal aliquots, which were processed and stored in three ways immediately after collection.(i) For snap-freezing in liquid nitrogen, the first aliquot was mixed with an equal volume of 80% glycerol in H2O and dipped into liquid nitrogen for a while.(ii) For enzyme inhibition, the second aliquot was mixed 10:1 with an enzyme inhibitor cocktail consisting of pefabloc, leupeptin, and aprotinin (Roche Diagnostics, Mannheim, Germany) at 25, 0.5, and 0.25 mg, respectively, in 5 ml of PBS with 50 mg of bovine serum albumin and 20 mg of EDTA. The samples with enzyme inhibitors were stored at +4°C.(iii) For plain storage, the third aliquot was stored immediately at +4°C without any processing. At the end of the working day (4 to 8 h after handling), all samples were placed in a −70°C freezer and stored for approximately 1 week before the analyses.
Sample dilutions.
Given different volumes of OF and different existing dilutions in buffer, we created a spreadsheet algorithm directing further dilutions in FCS-PBS with CPS to achieve a 1:10 dilution of neat saliva for assay and preincubation with CPS at 10 μg/ml for 30 min at room temperature. CPS was used in the assay to neutralize antipneumococcal CPS antibodies. The algorithm was based on the following assumptions: (i) that the pastette and unstimulated samples contained undiluted saliva; (ii) that the proportion of saliva in the Oracol and OraSure samples was calculated by subtraction of the known buffer volume; (iii) that the concentration of OF in the samples stored by snap-freezing was half that in the samples stored by other methods because of glycerol dilution; and (iv) that the volume of enzyme additive, 10% of the sample volume, in enzyme inhibition samples did not produce a significant dilution and was ignored. For several specimens, the existing dilution of saliva in the OraSure buffer was already greater than 1:10, so all OraSure specimens were assayed at a 1:16.7 dilution of saliva to buffer.
IgA enzyme immunoassay (EIA).
Microtiter plates (catalog no. 3591; Costar, Cambridge, Mass.) were coated with a 15 μg/ml concentration of CPSs 1, 5, 6B, and 14 (American Type Culture Collection, Manassas, Va.) or with a 5 μg/ml concentration of pneumococcal surface adhesin A (PsaA) (Aventis Pasteur, Toronto, Canada) diluted in PBS and incubated overnight at room temperature. Plates coated only with PBS were used as a background control to determine nonspecific binding. The PBS plates were treated in the same way as the antigen plates during the assay. All of the plates were blocked with 10% FCS-PBS for 1 h at +37°C and then emptied without being washed. The frozen OF samples were centrifuged for 10 min at 13,000 rpm, during which they thawed, and were diluted in FCS-PBS containing CPS according to the algorithm above. Human serum 89-SF (U.S. Food and Drug Administration, Bethesda, Md.) and an in-house serum with a high anti-PsaA concentration were used as reference sera for the CPS and PsaA assays, respectively. The samples were assayed at a single dilution in triplicate, and the reference sera were assayed at five serial threefold dilutions in duplicate. The samples were aliquoted (50 μl/well) and incubated for 2 h at room temperature with horizontal rotation (200 rpm). Mouse monoclonal anti-human IgA (HP6123; Centers for Disease Control and Prevention, Atlanta, Ga.), diluted 1:2,000 in FCS-PBS, was incubated for 2 h at room temperature, and then rabbit polyclonal phosphatase-conjugated anti-mouse IgG (315-055-045; Jackson's Immuno Research Laboratories, West Grove, Pa.) was incubated in FCS-PBS overnight at room temperature (50 μl/well). The substrate, p-nitrophenyl phosphate disodium (Sigma Immuno Chemicals, St. Louis, Mo.) in phosphate buffer, pH 9.8, was incubated for 1 h at +37°C. Between steps 1 and 3, the plates were washed four times with PBS containing 0.05% Tween 20 (Merck); after overnight incubation, the plates were washed three times with PBS-0.05% Tween 20 and two times with deionized water. Absorbances were measured at 405 nm, and the results were expressed as optical densities after subtraction of the corresponding sample optical density value in a background control plate coated only with PBS.
Analysis.
Concentrations were calculated with the enzyme-linked immunosorbent assay (ELISA) program (B. D. Plikaytis, bdp 1@cdc.gov, Centers for Disease Control and Prevention) as arbitrary units, assuming a concentration of 100 for the reference serum. Antibody concentrations conformed to a normal distribution after logarithmic transformation. Concentrations less than the detection limit were excluded. Differences in log concentrations between groups were tested by using Student's t test or linear regression. The analysis was conducted with Stata software (Statacorp, College Station, Tex.). Although separate studies were performed to evaluate laboratory storage and specimen collection, the data from the two studies were combined into a single regression model for the analysis of each explanatory variable. To overcome the uneven distribution of this data and to control for variation attributable to differences between the subjects, the subject number was introduced into the model as a fixed effect. Interactions were tested by using a Wald test of the combination of dummy variables representing the interaction terms.
RESULTS
We collected saliva specimens from 30 subjects by each of four different methods, for a total of 120 specimens. Each specimen was processed by the three different storage methods, yielding a total of 360 samples. We first examined the effects of storage methods on 30 specimens (one from each subject); 7 had been collected with the OraSure device, 7 had been collected by the pastette method, and 8 each had been collected by the Oracol method and the unstimulated-drooling method. In this small specimen set, the highest concentrations of IgA were observed in samples snap-frozen in liquid nitrogen. We therefore decided to undertake the evaluation of different collection methods by comparing primarily the samples that had been processed by snap-freezing (number of samples, 120; samples collected by each method, 30). On one day the EIA failed to meet quality criteria, and as the 24 samples for this EIA were exhausted, we performed repeat assays on samples from the same specimens. These samples were collected in the same way but were processed and stored by the plain method rather than by being snap-frozen.
According to the protocol discussed above, we should have 150 serotype-specific assay results from enzyme-treated samples (30), 270 results from plain samples (54), and 480 results from snap-frozen samples (96). However, not every sample had sufficient volume for all five assays and the actual numbers of assays completed were 148, 259, and 466, respectively. Of these, 215 assays were performed on samples collected by the OraSure method, 228 were collected by the Oracol method, 201 were collected by the pastette method, and 229 were collected by the unstimulated-drooling technique. Fifty-nine of these 873 assays resulted in concentrations less than the detection limit, and the analyses of concentration presented below are based on the full data set of the remaining positive values (n = 814).
The volume of specimens collected by the OraSure method is determined predominantly by the volume of the proprietary buffer. Compared with that of the OraSure specimens, the mean volume of the specimens collected by the pastette method was low and the mean volumes of those collected by the unstimulated-drooling technique and by the Oracol method were high (Table 1) (P < 0.0005; 95% confidence interval [CI], 0.58 to 0.79) for plain samples and 0.71 (P < 0.0005; 95% CI, 0.60 to 0.84) for enzyme-inhibited samples. For the anti-PsaA EIA, the ratios of the geometric mean concentrations relative to that of the snap-frozen samples were 1.30 (P = 0.11; 95% CI, 0.94 to 1.81) for the plain samples and 1.44 (P = 0.024; 95% CI, 1.05 to 1.98) for the enzyme-inhibited samples.
TABLE 1.
Mean, minimum, and maximum volumes of specimens taken by four different collection methods
| Collection method | Sample vol (μl)
|
||
|---|---|---|---|
| Mean | Minimum | Maximum | |
| OraSure | 1,105 | 200 | 1,400 |
| Oracol | 1,580 | 1,200 | 2,000 |
| Pastette | 673 | 150 | 1,500 |
| Unstimulated drooling | 1,405 | 550 | 2,000 |
| Total | 1,191 | 150 | 2,000 |
The geometric mean anti-S. pneumoniae IgA concentrations are shown for each of the five EIAs by each collection method in Table 3. (P = 0.44; 95% CI, 0.78 to 1.12) for the Oracol specimens, 0.84 (P = 0.07; 95% CI, 0.70 to 1.01) for the pastette specimens, and 0.98 (P = 0.81; 95% CI, 0.82 to 1.17) for the unstimulated specimens. For the anti-PsaA EIA, the ratios of the geometric mean concentrations relative to that of the OraSure specimens were 0.52 (P = 0.001; 95% CI, 0.36 to 0.76) for the Oracol specimens, 0.77 (P = 0.21; 95% CI, 0.52 to 1.16) for the pastette specimens, and 0.71 (P = 0.08; 95% CI, 0.49 to 1.04) for the unstimulated specimens.
TABLE 3.
Unadjusted geometric mean concentrations of anti-S. pneumoniae IgA by antibody specificity and collection method
| Collection method | Geometric mean concn (EIA units) of IgA specific for:
|
||||
|---|---|---|---|---|---|
| CPS 1 | CPS 5 | CPS 6B | CPS 14 | PsaA | |
| OraSure | 2.25 | 1.70 | 2.51 | 4.13 | 0.73 |
| Oracol | 1.82 | 1.36 | 1.90 | 3.85 | 0.31 |
| Pastette | 1.84 | 1.22 | 2.19 | 3.21 | 0.49 |
| Unstimulated drooling | 2.71 | 1.37 | 2.24 | 3.37 | 0.34 |
Analysis of the polysaccharide EIA results revealed a third significant interaction (P = 0.003) between the collection method and the storage method in the (natural log) concentrations of antibody detected. To illustrate this, the exponentials of the interaction coefficients have been collated in Table 4 to show how combinations of different collection and storage methods result in EIA antipolysaccharide IgA concentrations that differ from EIA antipolysaccharide IgA in samples collected by the OraSure method and snap-frozen in the laboratory (baseline concentrations). The differential effect of laboratory storage methods is more prominent in specimens taken by the pastette or by the unstimulated-drooling technique than in those taken with proprietary collection devices.
TABLE 4.
Ratios of concentrations of anti-S. pneumoniae IgA determined by various EIAs to that in snap-frozen OraSure specimens (baseline) relative to the method of storage
| Storage method | Ratio of anti-S. pneumoniae IgA concn by indicated collection method to concn in snap-frozen OraSure specimen
|
|||
|---|---|---|---|---|
| OraSure | Oracol | Pastette | Unstimulated drooling | |
| Snap-freezing | 1.00 | 1.06 | 1.01 | 1.34 |
| Enzyme inhibition | 0.93 | 0.78 | 0.76 | 0.60 |
| Plain | 1.06 | 0.71 | 0.59 | 0.42 |
Median times to collect specimens from study subjects were 1 min for the Oracol method, 2 min for the OraSure method, and 4 min each for the pastette and unstimulated-drooling techniques. The geometric mean times from starting the collection to completing the laboratory storage were 13.2 min for the OraSure specimens, 18.3 for the Oracol specimens, 5.3 for the pastette specimens, and 6.4 for the unstimulated specimens.
DISCUSSION
When saliva samples were taken from the same individuals by different field collection methods in random order, no significant differences in the anti-S. pneumoniae IgA antibody concentrations were detected. In a previous study, the Oracol method was shown to yield higher total concentrations of IgM and IgG antibodies than the OraSure method (20); this does not appear to apply to IgA. In accordance with a previous study (2), we found that laboratory storage methods that did not include promptly freezing the specimens led to a diminution of measurable IgA by approximately 30%. The statistically significant interaction noted between collection and storage methods may be explained in terms of processing times. Although collection by the OraSure and Oracol methods is approximately 2 to 3 min quicker than that by the drooling or pastette technique, the specimen must then be extracted by centrifugation, adding in our protocol 5 and 10 min, respectively, to the delay before storage. In such specimens, the superiority of snap-freezing was less apparent (Table 4), which suggests that whatever the advantage of snap-freezing is, it is lost if not applied soon after the saliva has left the mouth.
Methodological differences in both the collection and storage of saliva specimens seem to affect assays of pneumococcal antibodies differently depending on whether their target is protein (PsaA) or a polysaccharide capsule. The concentrations of anti-PsaA IgA in specimens obtained by the Oracol method were significantly lower than in specimens obtained by the OraSure method (Table 3), and laboratory treatment with proteolytic enzyme inhibitors produced significantly higher concentrations than did snap-freezing (Table 2). The concentrations of anti-PsaA in OF were very low both in comparison to the concentration in the serum control and in terms of optical density—half of all the specimens registering concentrations less than the detection limit came from one anti-PsA EIA. This may be due to the high background of the anti-PsaA EIA. Because these results are probably less robust than those for the anticapsular IgA EIAs, the remainder of the discussion relates to anticapsular antibodies alone.
TABLE 2.
Unadjusted geometric mean concentrations of anti-S. pneumoniae IgA by antibody specificity and laboratory storage method
| Storage method | Geometric mean concn (EIA units) of IgA specific for:
|
||||
|---|---|---|---|---|---|
| CPS 1 | CPS 5 | CPS 6B | CPS 14 | PsaA | |
| Snap-freezing | 2.61 | 1.70 | 2.29 | 4.47 | 0.39 |
| Enzyme inhibition | 1.82 | 1.26 | 2.03 | 2.83 | 0.55 |
| Plain | 1.65 | 1.04 | 2.11 | 2.91 | 0.51 |
Specimens obtained by the drooling or pastette technique are simply saliva, whereas the target of the OraSure and Oracol devices is the crevicular fluid which seeps out from the gum margin. Most of the volume of these device-collected specimens consists of the buffer used to extract this fluid from the pad or sponge. On average, the final specimen volumes exceeded buffer volumes by only 200 μl for the OraSure method and 600 μl for the Oracol method. Estimating the true amount of saliva by subtracting the volume of the buffer, we assayed saliva collected by the OraSure device at a dilution of 1:16.7 and all other specimens at a dilution of 1:10. Arguably, the OraSure results should be multiplied by 1.67 to compensate for this difference in the actual volume of the saliva. However, the reason for this differential at the protocol stage was that saliva in the OraSure technique is already diluted 1:2 to 1:15 in its own proprietary buffer and requires further dilution in CPS. The alternative approach, assaying the OraSure specimen whole as if it were neat saliva, would have yielded IgA concentrations between 1/2 and 1/15 of those recorded here. The compromise approach adopted in our protocol produced specimen volumes by each collection method that were sufficient to undertake five EIAs after incubation in CPS, and the concentrations of antibody in the specimens of each method were broadly equivalent. Given this broad equivalence, the choice of collection methods is likely to be determined more by operational or economic considerations than by antibody yield.
According to our study, the best method for storing saliva specimens before testing them for anticapsular IgA antibodies is snap-freezing in liquid nitrogen. This yields an antibody concentration 41 to 47% higher than that in specimens temporarily stored at +4°C (whether or not the sample was stored with protease inhibitors), but only if the samples can be frozen within a few minutes of leaving the mouth, which requires both a rapid collection technique and the presence of a liquid nitrogen flask close to the point of collection. Freezing in dry ice is probably a practical choice if liquid nitrogen or a −70°C freezer is not available when samples are collected.
In conclusion, to maximize anti-S. pneumoniae IgA antibody concentrations in saliva samples, we suggest freezing them as soon as possible and storing them at −70°C. The collection method has a minor effect on antibody concentration, and the choice between different collection methods can be based on several criteria: ease of sampling, unit cost, and the volume of the specimen. The collection of drool without stimulation is an inexpensive, relatively time-consuming, but easy and practical method provided that the subject is cooperative. Drooling cannot be used with infants. Obtaining a specimen with a pastette is tedious, requires approximately 4 min of the investigator's time, and yields smaller average volumes. The OraSure and Oracol devices are both quick and easy to use, but they are expensive at $5.00 and $0.80 per specimen, respectively. Thus, the choice of collection methods for each study depends on practical rather than scientific considerations.
(P was <0.05 for all six pairwise comparisons). The order in which the collection methods were performed did not affect the sample volume. Furthermore, restriction of the analysis to the first sample taken from each subject yielded the same rank order of the volumes. The mean volume of the samples taken from 9 women (1,214 μl) was indistinguishable from that obtained from 21 men (1,181 μl).
After log transformation, the distribution of IgA antibody concentrations was normal. Unadjusted geometric mean IgA antibody concentrations are shown for each of the five type-specific EIAs by each storage method in Table 2. Snap-frozen samples had higher concentrations than those processed by the other two methods in all EIAs except in that with anti-PsaA. In the regression model of log concentrations, there was a significant interaction (P = 0.014) between type-specific EIA and storage method, but this was extinguished when the analysis was restricted to the polysaccharide EIAs alone (P = 0.56). Therefore, the data for the anti-PsaA EIA and those for the antipolysaccharide EIAs were analyzed separately.
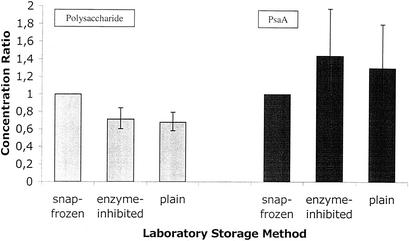
In the analysis of antipolysaccharide antibodies, after adjustment for subject, collection method, and serotype, the ratios of the geometric mean concentrations relative to that of the snap-frozen samples were 0.68 (Fig. 1) Collection by the OraSure method was superior to that of the other three methods for all EIAs; however, in the regression model, another significant interaction (P = 0.04) occurred between the EIA type and the collection methods. Once again, this interaction disappeared when the analysis was restricted to the polysaccharide EIAs alone (P = 0.58). The results of the anti-PsaA EIA and those of the antipolysaccharide EIAs were also analyzed separately.
FIG. 1.
Concentration ratios for polysaccharide (gray bars)- and PsaA (black bars)-specific IgA antibodies in saliva specimens by laboratory storage method.
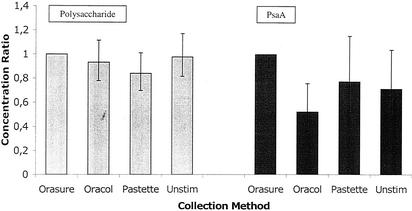
In the analysis of antipolysaccharide antibodies, after adjustment for subject, laboratory storage method, and serotype, the ratios of the geometric mean concentrations relative to that of the OraSure specimens were 0.93 (Fig. 2)
FIG. 2.
Concentration ratios for polysaccharide (gray bars)- and PsaA (black bars)-specific IgA antibodies in saliva specimens by the indicated collection method. Unstim, unstimulated drooling.
Acknowledgments
We thank all the volunteers who took part in the study and James Nokes for critical reading of the manuscript.
J.A.G.S. is supported by a fellowship from The Wellcome Trust of Great Britain (no. 061089).
This paper is published with the permission of the Director of the Kenya Medical Research Institute.
REFERENCES
- 1.Adegbola, R. A., E. K. Mulholland, O. Secka, S. Jaffar, and B. M. Greenwood. 1998. Vaccination with a Haemophilus influenzae type b conjugate vaccine reduces oropharyngeal carriage of H. influenzae among Gambian children. J. Infect. Dis. 177: 1758-1761. [DOI] [PubMed] [Google Scholar]
- 2.Butler, J. E., J. E. Spradling, J. Rowat, J. Ekstrand, and S. J. Challacombe. 1990. Humoral immunity in root caries in an elderly population. 2. Oral. Microbiol. Immunol. 5:113-120. [DOI] [PubMed] [Google Scholar]
- 3.Challacombe, S. J., and P. J. Shirlaw. 1999. Immunology of diseases of the oral cavity, p. 1313. In P. L. Ogra, J. Mestecky, M. E. Lamm, W. Storober, J. Bienenstock, and J. R. McGhee (ed.), Mucosal immunology, 2nd ed. Academic Press, London, United Kingdom.
- 4.Choo, S., Q. Zhang, L. Seymour, S. Akhtar, and A. Finn. 2000. Primary and booster salivary antibody responses to a 7-valent pneumococcal conjugate vaccine in infants. J. Infect. Dis. 182: 1260-1263. [DOI] [PubMed] [Google Scholar]
- 5.Dagan, R., M. Muallem, R. Melamed, O. Leroy, and P. Yagupsky. 1997. Reduction of pneumococcal nasopharyngeal carriage in early infancy after immunization with tetravalent pneumococcal vaccines conjugated to either tetanus toxoid or diphtheria toxoid. Pediatr. Infect. Dis. J. 16:1060-1064. [DOI] [PubMed] [Google Scholar]
- 6.Granade, T. C., S. K. Phillips, B. Parekh, C.-P. Pau, and J. R. George. 1995. Oral fluid as a specimen for detection and confirmation of antibodies to human immunodeficiency virus type 1. Clin. Diagn. Lab. Immunol. 2:395-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kauppi, M., J. Eskola, and H. Käyhty. 1995. Anti-capsular polysaccharide antibody concentrations in saliva after immunization with Haemophilus influenzae type b conjugate vaccines. Pediatr. Infect. Dis. J. 14:286-294. [DOI] [PubMed] [Google Scholar]
- 8.Kauppi, M., L. Saarinen, and H. Käyhty. 1993. Anti-capsular polysaccharide antibodies reduce nasopharyngeal colonization by Haemophilus influenzae type b in infant rat. J. Infect. Dis. 167: 365-371. [DOI] [PubMed] [Google Scholar]
- 9.Kilian, M., J. Reinholdt, H. Lomholt, K. Poulsen, and E. V. G. Frandsen. 1996. Biological significance of IgA1 proteases in bacterial colonization and pathogenesis: critical evaluation of experimental evidence. APMIS 104:321-338. [DOI] [PubMed] [Google Scholar]
- 10.Korkeila, M., H. Lehtonen, H. Åhman, O. Leroy, J. Eskola, and H. Käyhty. 2000. Salivary anti-capsular antibodies in infants and children immunised with Streptococcus pneumoniae capsular polysaccharides conjugated to diphtheria or tetanus toxoid. Vaccine 18:1218-1226. [DOI] [PubMed] [Google Scholar]
- 11.Malley, R., A. M. Stack, M. L. Ferretti, C. M. Thompson, and R. A. Saladino. 1998. Anticapsular polysaccharide antibodies and nasopharyngeal colonization with Streptococcus pneumoniae in infant rats. J. Infect. Dis. 178: 878-882. [DOI] [PubMed] [Google Scholar]
- 12.Mbelle, N., R. E. Huebner, A. D. Wasas, A. Kimura, I. Chang, and K. P. Klugman. 1999. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J. Infect. Dis. 180: 1171-1176. [DOI] [PubMed] [Google Scholar]
- 13.Millar, E. V., K. L. O'Brien, O. S. Levine, S. Kvamme, R. Reid, and M. Santosham. 2000. Toward elimination of Haemophilus influenzae type b carriage and disease among high-risk American Indian children. Am. J. Public Health 90:1550-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mortimer, P. P., and J. V. Parry. 1988. The use of saliva for viral diagnosis. Epidemiol. Infect. 101:197-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nieminen, T., J. Eskola, and H. Käyhty. 1998. Pneumococcal conjugate vaccination in adults: circulating antibody secreting cell response and humoral antibody responses in saliva and in serum. Vaccine 16:630-636. [DOI] [PubMed] [Google Scholar]
- 16.Nurkka, A., H. Åhman, M. Korkeila, V. Jäntti, H. Käyhty, and J. Eskola. 2001. Serum and salivary anti-capsular antibodies in infants and children immunized with the heptavalent pneumococcal conjugate vaccine PncCRM. Pediatr. Infect. Dis. J. 20:25-33. [DOI] [PubMed] [Google Scholar]
- 17.Nurkka, A., H. Åhman, M. Yaich, J. Eskola, and H. Käyhty. 2001. Serum and salivary anti-capsular antibodies in infants and children vaccinated with octavalent pneumococcal conjugate vaccines, PncD and PncT. Vaccine 20:194-201. [DOI] [PubMed] [Google Scholar]
- 18.Nurkka, A., J. MacLennan, V. Jäntti, S. Obaro, B. Greenwood, and H. Käyhty. 2000. Salivary antibody response to vaccination with meningococcal A/C polysaccharide vaccine in previously vaccinated and unvaccinated Gambian children. Vaccine 19:547-556. [DOI] [PubMed] [Google Scholar]
- 19.Sloyer, J. L., Jr., V. M. Howie, J. H. Ploussard, G. Schiffman, and R. B. Johnston, Jr. 1976. Immune response to acute otitis media: association between middle ear fluid antibody and the clearing of clinical infection. J. Clin. Microbiol. 4:306-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vyse, A. J., B. J. Cohen, and M. E. Ramsay. 2001. A comparison of oral fluid collection devises for use in the surveillance of virus diseases in children. Public Health 115:201-207. [DOI] [PubMed] [Google Scholar]