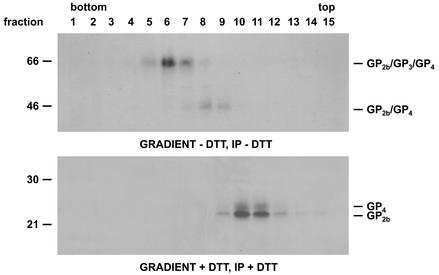
FIG. 8.
Stability of the GP2b/GP4 and GP2b/GP3/GP4 complexes after disulfide bond disruption. Concentrated [35S]cysteine-labeled EAV particles were dissolved in NT-0.4% Triton (pH 7.4) in the presence or absence of 5 mM DTT and loaded onto a 5 to 20% (wt/wt) sucrose density gradient. After centrifugation for 52 h at 38,000 rpm and 4°C in an SW41 rotor, fractions were collected and subjected to immunoprecipitations (IP) with a mixture of αGP2b and αGP4 in the presence (lower panel) or absence (upper panel) of 5 mM DTT. The immunoprecipitates were analyzed by SDS-PAGE under reducing (lower panel) or nonreducing (upper panel) conditions. Fraction numbers are indicated above the autoradiographs. The numbers on the left are the molecular sizes, in kilodaltons, of marker proteins analyzed in the same gel.