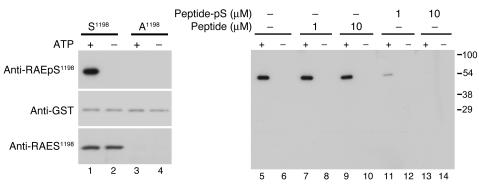
Figure 3. Characterization of phosphomotif-specific antibody targeting RAEpS1198 .
Immunoblots of in vitro phosphorylation reactions of GST–α1H II-III loop fusion proteins (1 μM) — wild-type (S1198) and mutant (A1198) — catalyzed by 10 nM CaMKIIγC (Ca2+ [0.5 mM], CaM [2 μM] ± ATP [100 μM]) showing selective recognition of pS1198 by anti-RAEpS1198 (top row, lanes 1, 5, 7, 9). Below is an anti-GST immunoblot for each fusion, indicating equal loading (middle row, lanes 1–4). Notably, anti-RAES1198 recognizes S1198 without regard for phosphorylation state (bottom row, lanes 1 and 2). Anti-RAEpS1198 preadsorption with phosphopeptide (Peptide-pS, lanes 11–14) but not non-phosphopeptide (Peptide, lanes 7–10) suppressed anti-RAEpS1198 immunoreactivity, showing phosphorylation-dependent interaction, confirming phosphomotif specificity.