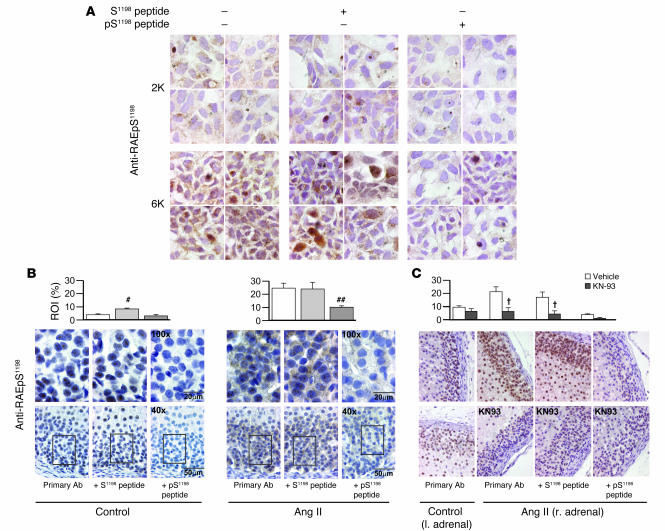
Figure 6. Phosphorylation-state of Ser1198 in α1H channels in situ and in vivo.
Immunohistochemical detection of pS1198 in: CaMKIIγC-transfected α1H/mTREK double-stable cells following 6 mM K+ depolarization (1 minute) (A); thin sections of rat adrenal glands harvested 30 minutes after vehicle or Ang II infusion, 50–200 ng/kg/min (n = 4 and 7 animals, respectively) (B); thin sections of rat adrenal glands subcapsularly perfused (1 μl/min) with D5W with or without 100 μM KN-93 30 minutes before and during a 30-minute systemic infusion of Ang II at 50 ng/kg/min in uniadrenalectomized rats (n = 3 animals each) (C). Anti-RAEpS1198 immunohistochemistry revealed DAB immunostaining in the subcapsular ZG (B and C). Note the increase in signal strength after stimulation (A–C). Signal competed with an 80-fold molar excess of antigenic phosphopeptide, pS1198 peptide, but not non-phosphopeptide, S1198 peptide, evaluated at either ×40 (B, bottom row; and C) or ×100 (B, top row; and A). All samples were counterstained with hematoxylin. Quantification of ZG DAB immunostaining expressed as percent of ROI. #P < 0.05, control S1198 peptide versus antibody alone. ##P < 0.05, stimulated pS1198 peptide versus antibody alone (ANOVA on ranks, Student-Newman-Keuls method). †P < 0.05, KN-93 versus vehicle, infused using the primary antibody alone or with pS1198 peptide preadsorption (ANOVA, Student-Newman-Keuls method).