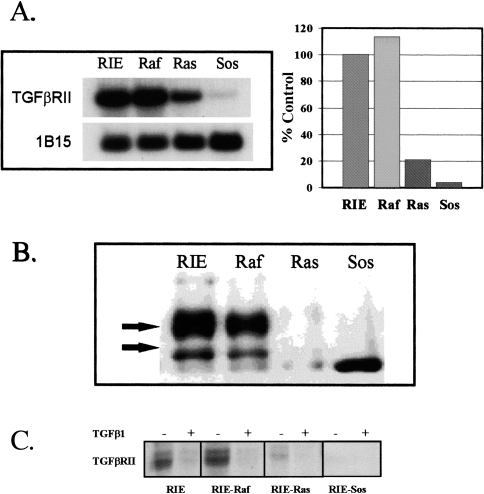
Figure 3.
(A) TGFβRII mRNA expression in parental and transformed RIE-1 cells. Poly(A) RNA was isolated from subconfluent monolayers. Four micrograms of RNA were loaded per lane. Northern blots were prepared and probed with radiolabeled cDNA, as described in Materials and Methods. 1B15 was used as a constitutive probe to control for loading. This experiment was repeated at least four times. Results are expressed as a percentage of mRNA expression in parental RIE-1 cells, as determined by densitometry. (B) TGFβRII protein levels in parental and transformed RIE-1 cells. Protein lysates were prepared from subconfluent cells. Immunoprecipitations and Western blotting were done as described in Materials and Methods. The antibody is a rabbit polyclonal anti-TGFβRII antibody. The arrows indicate the two primary processed forms of TGFβRII [37,38]. All signals were competed away by pre-incubation with a blocking peptide (not shown). (C) Cross-linking to surface TGFβRII. [125I]-TGFβ1 (NEN-Dupont) was added to a final concentration of 50 pM in the presence (+) or absence (-) of 500 pM TGFβ1 and the incubation continued for an additional two hours at 4°C. The ligand solution was removed, the cells washed four times with cold KRH and 0.1 mg/ml disuccinylsuberate in KRH was added for 15 minutes at 4°C. Autoradiograms were prepared as described in Materials and Methods.