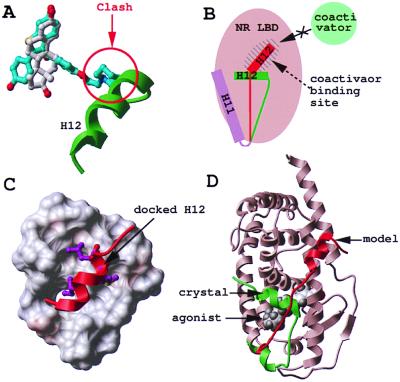
Figure 1.
Modeling of the antagonist-bound structure of RAR. Agonist (white) and antagonist (cyan) superimpose in the binding pocket of ERα, but the antagonist presents an additional protruding arm that pushes helix 12 (H12, green) away (A). As a result, H12 relocates in the coactivator binding pocket of the receptor (H12, red) (B). Based on the ERα structure, helix H12 of RARγ (red) was docked to the coactivator binding pocket of the RARγ-LBD (critical hydrophobic residues are displayed in magenta) (C), and the C terminus of the protein was remodeled from its agonist-bound conformation (green) to its antagonist-bound conformation (red) (D).