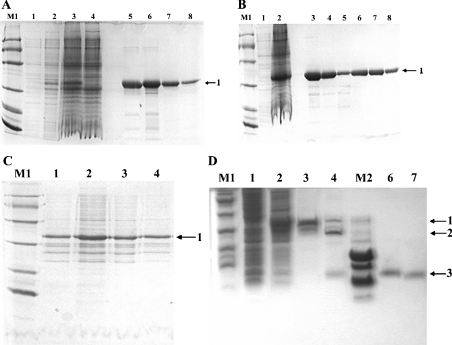
Figure 3. SDS/PAGE analysis of rLNTX3 and rLNTX1.
(A)–(C) employed Tris/glycine/SDS/16%-(w/v)-PAGE and (D) employed Tris/tricine/SDS/16%-PAGE. Lane M1, protein markers of low molecular mass (kDa): 97, 66, 43, 31, 20 and 14; lane M2, peptide markers (kDa): 16, 14, 10, 0.8, 0.6 and 0.2; bands labelled 1, 2 and 3 are fusion protein, GST-tag and recombinant peptides respectively. (A) Total proteins of uninduced and induced cultures together with GST–LNTX3 obtained from each of the purification steps. Lanes 1 and 2, total proteins of culture at 37 °C after 0 and 4 h induction respectively; lanes 3 and 4, total proteins of culture at 16 °C after 0 and 20 h induction respectively; lanes 5 and 6, elution fractions from the affinity column; lanes 7 and 8, purified GST–LNTX3 from the Superdex75 column. (B) Total proteins of uninduced and induced culture together with GST–LNTX1 obtained from each of the purification steps. Lanes 1 and 2, total proteins of culture at 16 °C after 0 and 20 h induction respectively; lanes 3–5, elution fractions from the affinity column; lanes 7 and 8, purified GST–LNTX1 from the Superdex75 column. (C) Proteins from supernatant and pellet of lysate after the induction of IPTG for 20 h at 16 °C. Lanes 1 and 2, the pellet and supernatant of LNTX1 respectively; lanes 3 and 4, the pellet and supernatant of LNTX3 respectively. (D) Cleavage of fusion protein and purified recombinant peptides. Lanes 1 and 2, total proteins of uninduced and induced culture of LNTX1; lanes 3 and 4, GST–LNTX1 after being mixed with thrombin for 0 and 8 h; lanes 6 and 7, the purified rLNTX1 and rLNTX3 respectively.