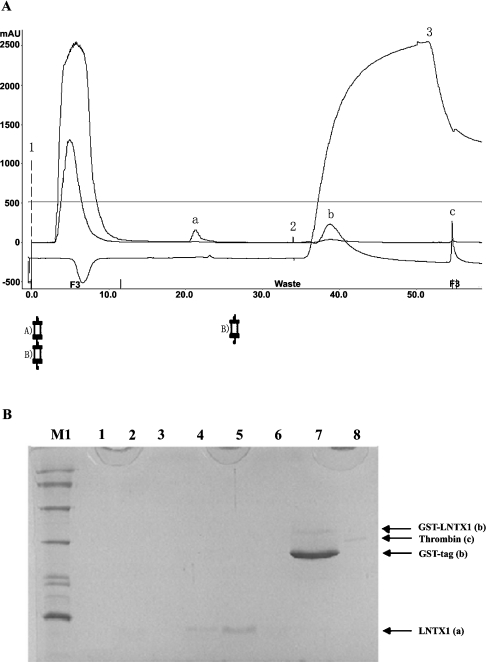
Figure 4. Purification of recombinant peptides on a benzamidine FF column placed in series below the GSTrap FF column.
(A) Chromatography of rLNTX1. Position 1 indicates the sample application. Positions 2 and 3 show the beginning of the high-salt elution and low-pH-buffer elution respectively. The high-salt-wash buffer contained 20 mM sodium phosphate and 1.0 M NaCl, pH 7.0; the low-pH-wash buffer contained 10 mM HCl and 0.5 M NaCl, pH2.0. Column A was a GSTrap FF 1-ml-volume column; column B was a HiTrap Benzamidine FF 1 ml column; ‘a’ is the released rLNTX1 peptide; ‘b’ is a mixture of GST-tag and some non-cleaved fusion proteins; ‘c’ is bovine thrombin. Abbreviation: mAU, milli-absorbance unit. (B) Tris/glycine/SDS/16%-PAGE analysis of each peak in the above chromatography. Lane M1, protein markers of low molecular mass; lanes 1–3, flow-through during sample loading; lanes 4–6, peak ‘a’ in (A); lane 7, peak ‘b’ in (A); lane 8, A chain (31 kDa) of bovine thrombin (‘c’ in A).