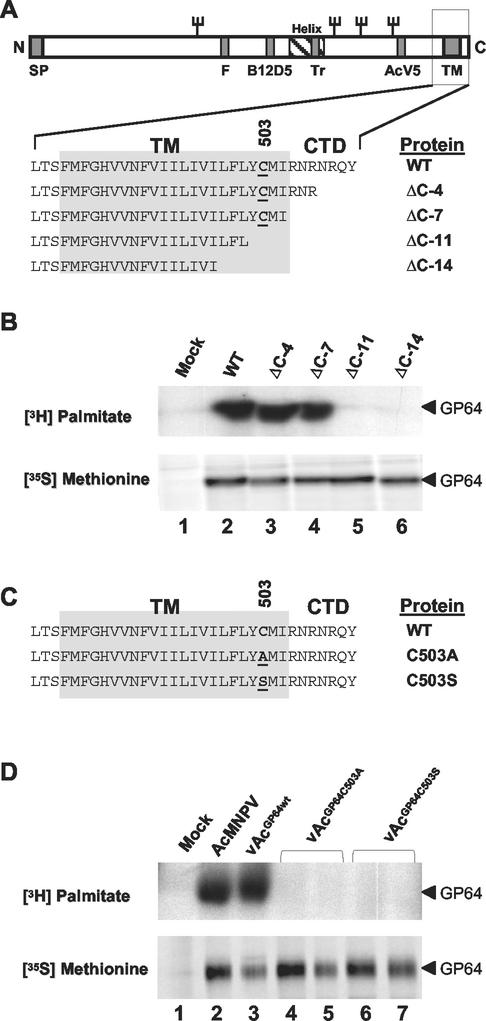
FIG. 1.
Palmitoylation of truncated or substituted GP64 proteins. (A) A diagrammatic representation of the GP64 protein shows the locations of the signal peptide (SP), fusion domain (F), epitopes (B12D5 and AcV5), predicted amphipathic alpha helix (Helix), trimerization domain (TR), transmembrane domain (TM), and cytoplasmic tail domain (CTD). The names of GP64 constructs containing deletions in the CTD and TM domain are indicated on the right, and Cys503 is underlined. (B) GP64 labeling with [3H]palmitate or [35S]methionine. Cells infected with wild-type AcMNPV (WT) or recombinant viruses expressing truncated forms of GP64 (ΔC-4, ΔC-7, ΔC-11, and ΔC-14) were pulse-labeled with [3H]palmitate or [35S]methionine, and then GP64 was immunoprecipitated with MAb AcV1. Results from [3H]palmitate labeling are shown in the upper panel, and those from [35S]methionine labeling are shown in the lower panel. (C) Sequences of the transmembrane and cytoplasmic tail domain of AcMNPV GP64 proteins. The wild-type AcMNPV (WT) sequence is shown above sequences of two mutant GP64 proteins containing substitutions at Cys503 (C503A and C503S). Substitutions are indicated by underlined amino acids. (D) GP64 labeling with [3H]palmitate or [35S]methionine and immunoprecipitation were performed as described for panel B. Cells were infected with viruses expressing wild-type GP64 (AcMNPV and vAcGP64wt) or GP64 proteins with substitutions at Cys503 (vAcGP64C503A and vAcGP64C503S). The lower panel shows GP64 labeling with [35S]methionine, and the upper panel shows labeling with [3H]palmitate. For immunoprecipitations from cells infected with vAcGP64C503A and vAcGP64C503S, duplicate infection and immunoprecipitation experiments are shown (lanes 4 and 5 and 6 and 7, respectively).