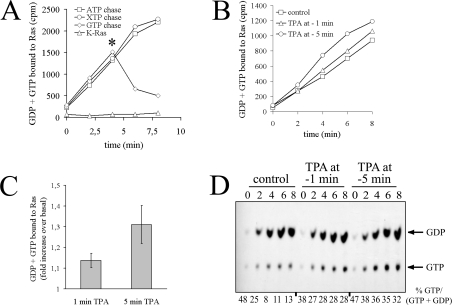
Figure 2. PMA stimulates nucleotide exchange on Ras.
(A) Permeabilization assay for assessment of nucleotide exchange on Ras. Serum-starved COS-7 cells were permeabilized with streptolysin O in the presence of [α-32P]GTP. Ras proteins were immunoprecipitated at various time points with the Y13-259 antibody in the presence or absence of 5 μg/ml recombinant K-Ras protein, as indicated. [α-32P]GTP incorporation was chased by addition of 100 μM of indicated purine nucleotides at the time point marked by an asterisk. Washed immunoprecipitates were subjected to Cerenkov counting. (B) PMA (‘TPA’) elevates nucleotide exchange on Ras. Serum-starved COS-7 cells were challenged with 100 nM PMA 5 or 1 min prior to permeabilization in the presence of [α-32P]GTP and quenching of the first assay point (zero time as recorded). Ras proteins were immunoprecipitated with Y13-259 at the indicated time points and radioactivity associated with immunoprecipitates was evaluated by Cerenkov counting. (C) Quantification of results presented in (B). The amount of GDP + GTP bound to Ras 6 min after quenching the zero-time assay point (as recorded in B) was plotted as the fold increase of radioactivity bound to Ras in PMA-stimulated cells versus unstimulated cells. Results shown represent the means±S.E.M. for at least three independent experiments. (D) Separation of guanine nucleotides associated with Ras. Guanine nucleotides were eluted from the same immunoprecipitates as in (B) and separated by TLC. Values for % GTP/(GDP + GTP) are given below the Figure. Three independent experiments yielded similar results.