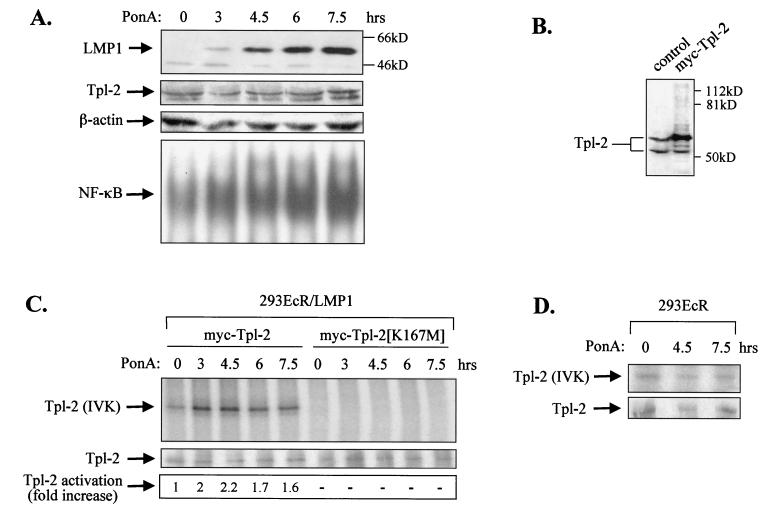
FIG. 2.
LMP1 promotes the activation of Tpl-2. (A) Induction of LMP1 in 293EcR/LMP1 cells carrying an ecdysone-regulatable LMP1, following addition of a 10 μM concentration of the ecdysone analogue ponasterone A (PonA; upper panel). Lysates from 293EcR/LMP1 cells treated with PonA or from untreated cultures were analyzed for endogenous Tpl-2 levels by using the anti-Tpl-2 polyclonal antibody M20 or for β-actin levels, as indicated. Nuclear extracts from parallel cultures were examined for NF-κB DNA binding activity by EMSA (lower panel). (B) HEK 293EcR/LMP1 cells were transfected with 0.3 μg of myc-tagged Tpl-2 per 106 cells and 24 h later were analyzed for Tpl-2 expression by immunoblotting, using the M20 anti-Tpl-2 polyclonal antibody (lane 2). Lysates from untransfected cultures (lane 1) were used to verify that, under these conditions, myc-Tpl-2 is expressed at near-physiological levels. (C) LMP1 promotes the activation of Tpl-2, as measured by enhanced autophosphorylation. HEK 293EcR/LMP1 cells were transfected with 0.3 μg of myc-tagged Tpl-2 (lanes 1 to 5) or its catalytically inactive mutant, Tpl-2[K167M] (lanes 6 to 10), and then stimulated with ponasterone A for 0, 3, 4.5, 6, or 7.5 h. Duplicate cultures from each time point were pulled together to minimize differences in transfection efficiencies and processed for in vitro kinase assays as described in Materials and Methods. Parallel anti-myc immunoprecipitates were probed for Tpl-2 using the M20 anti-Tpl-2 polyclonal antibody (middle panel). Relative levels of autophosphorylation were measured on a phosphorimager and are shown in the lower panel. (D) Parental HEK 293EcR cells were transiently transfected with 0.3 μg of myc-tagged Tpl-2 and then stimulated with 10 μM ponasterone A for 0, 4.5, or 7.5 h. Tpl-2 autophosphorylation was examined as described for panel C (upper panel). Anti-myc immunoprecipitates were also probed with the anti-Tpl-2 polyclonal antibody M20 (lower panel).