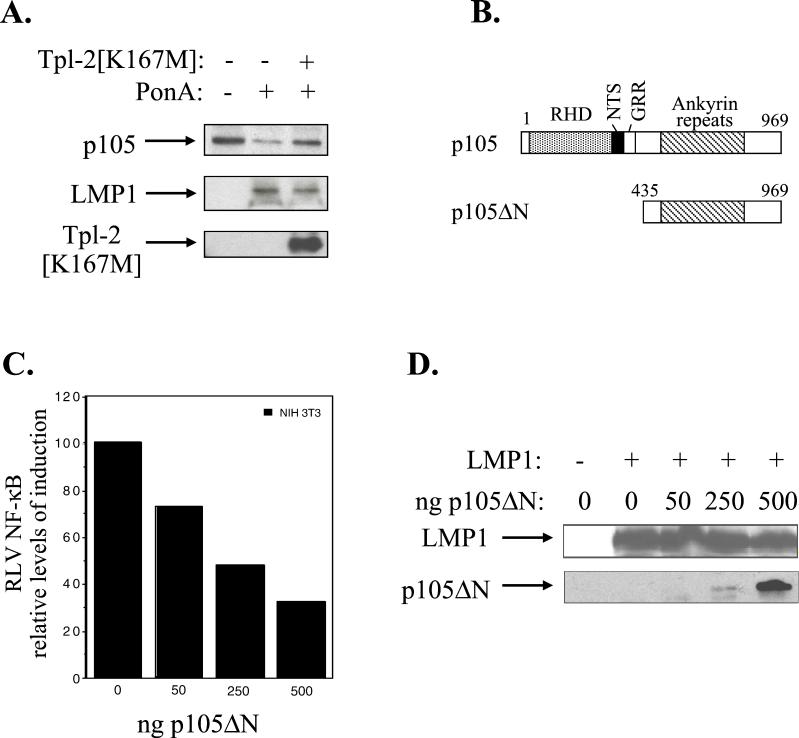
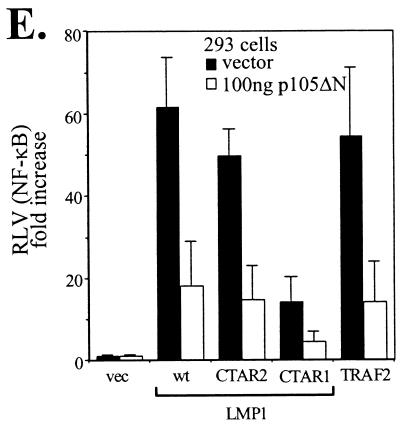
FIG. 6.
LMP1-induced Tpl-2-mediated p105 degradation contributes to NF-κB transactivation. (A) Kinase-inactive Tpl-2 inhibits LMP1-mediated p105 degradation. HA-tagged Tpl-2[K167M] (1 μg) and 3 μg of myc-tagged p105 were transfected in 293EcR/LMP1 cells. Following treatment with the ecdysone analogue ponasterone A for 9 h, cell lysates were isolated and analyzed for LMP1, Tpl-2, or myc-p105 expression by immunoblotting. (B) Structure of p105 and the N-terminus-deleted, nondegradable mutant used in this study. RHD, rel-homology domain; NTS, nuclear translocation signal; GRR, glycine-rich region. Both constructs carry a myc tag in their N termini. (C) Transfection of p105ΔN in NIH 3T3 cells inhibits LMP1-induced NF-κB activation. The relative decrease in NF-κB luciferase values (RLVs) is shown in histogram form, in which LMP1 has been given the arbitrary value of 100. The actual increase in NF-κB transactivation induced by LMP1 in this representative assay was 8.5-fold. (D) Identical lysates from the experiment shown in panel C were analyzed for LMP1 expression in order to verify that the inhibitory effects of p105ΔN on LMP1-induced NF-κB activation were not due to altered LMP1 levels (upper panel). Deleted p105 expression was assessed by immunoblot analysis using anti-myc tag 9E10 MAb (lower panel). (E) Transfection of a low plasmid concentration (100 ng) of p105ΔN significantly inhibits NF-κB transactivation induced by 1 μg of LMP1, LMP1 CTAR1 [LMP1Δ(332-386)], or LMP1 CTAR2 (LMP1AxAxA) in HEK 293 cells. The effects of this deleted p105 mutant on TRAF2-mediated NF-κB reporter activity are also shown. Data (RLVs) are the mean values (± SD) from three independent experiments.