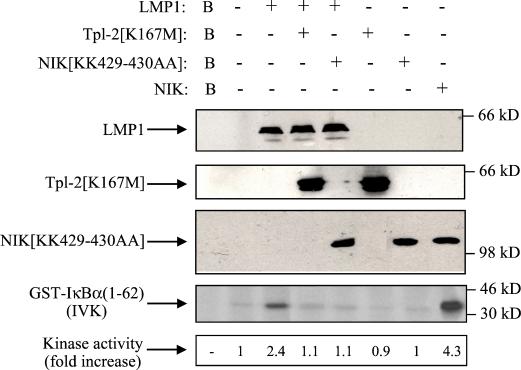
FIG. 7.
Involvement of Tpl-2 in LMP1-mediated IκBα activation. HEK 293 cells were transiently transfected with 2.5 μg of pSG5-LMP1 or control vector in the presence or absence of equal amounts of kinase-inactive Tpl-2 (lanes 4 and 6) or NIK (lanes 5 and 7). Lysates were subjected to immunoblot analysis for LMP1, Tpl-2, or NIK expression (upper three panels). Endogenous IKKα was immunoprecipitated using the H744 polyclonal anti-IKKα antibody (Santa Cruz Biotechnology) and analyzed for kinase activity in an in vitro kinase assay (IVK) using GST-IκBα(1-62) as the substrate. As a positive control, lysates from wild-type NIK-transfected cells were used (lane 8). IκBα phosphorylation was measured on a phosphorimager, and relative levels of kinase activity are shown. In three independent experiments, LMP1 induced a mean of 2.1 (±0.4)-fold increase in IκBα activation, while kinase-inactive Tpl-2 consistently suppressed this effect by more than 85%. Background phosphorylation (B, lane 1) from mock immunoprecipitation (in the absence of IKKα antibody) is also shown. Expression of wild-type Tpl-2 has been previously shown to induce IκBα phosphorylation in 293 cells (33).