Abstract
Statins have a variety of properties that are independent of their lipid lowering ability. These anti-inflammatory, antioxidant, immunomodulatory, and antiapoptotic features have been collectively referred to as pleiotropic effects. Severe sepsis is an intense infection-induced inflammatory syndrome that ultimately results in organ dysfunction. Because so many cascades are triggered during sepsis, merely blocking a single component may be insufficient to arrest the inflammatory process. A growing body of evidence suggests that statins may indeed have a protective effect against severe sepsis and reduce the rate of infection-related mortality. This novel primary prevention concept may have far-reaching implications for the future management of serious infections. Moreover, it was recently shown that statins potentially improve outcome after the onset of sepsis. The stage is now set for randomized clinical trials that will determine the precise role, if any, that statins may have in preventing and treating sepsis.
Severe sepsis is an infection-induced inflammatory syndrome that ultimately leads to organ dysfunction. It is estimated that more than 500,000 episodes of sepsis occur each year in the USA alone, and that 20–50% of these patients will die [1]. Disturbingly, the incidence of sepsis and number of sepsis-related deaths appear to be increasing [1]. Important progress has been made in recent years, and interventions such as activated protein C, early goal-directed therapy, and possibly low-dose corticosteroids have been shown to improve survival in patients with severe sepsis [2]. Despite these advances, mortality remains unacceptably high and care for patients with sepsis costs as much as $50,000 per patient, resulting in an economic burden of nearly $17 billion annually in the USA alone [1].
It is generally accepted that sepsis syndrome reflects the delicate balance between extensive triggering of defense mechanisms by invading micro-organisms and both direct and indirect effects of these micro-organisms and their products. Most investigators would agree that severe sepsis is accompanied by the inability to regulate the inflammatory response and that the cause of this perturbation is still not well defined [2]. In fact, Sir William Osler, some 100 years ago, suggested that, 'Except on few occasions the patient appears to die from the body's response to infection rather than from it' [3]. Over the past 3 decades, numerous trials have failed to demonstrate that blocking specific inflammatory mediators is beneficial in sepsis. Despite this frustration, new strategies are being explored such as blockade of high-mobility group B1 protein, macrophage migration inhibitory factor, and the complement split product C5a [2]. However, in complex situations such as severe sepsis, multiple cellular activation processes are involved and many humoral cascades are triggered, and so merely blocking a single component may be insufficient to arrest the inflammatory process [4]. Given the acute onset and unpredictable nature of sepsis, primary prevention has not been within the thinking paradigm of this syndrome.
Statins have a wide variety of properties that are independent of their lipid-lowering ability. These anti-inflammatory, anti-oxidant, immunomodulatory, antiapoptotic, antiproliferative, antithrombotic, and endothelium protecting features have been collectively referred to as pleiotropic effects [5]. A large and growing body of knowledge supports the notion that statins may be beneficial in preventing and possibly treating sepsis (Fig. 1) [4].
Figure 1.
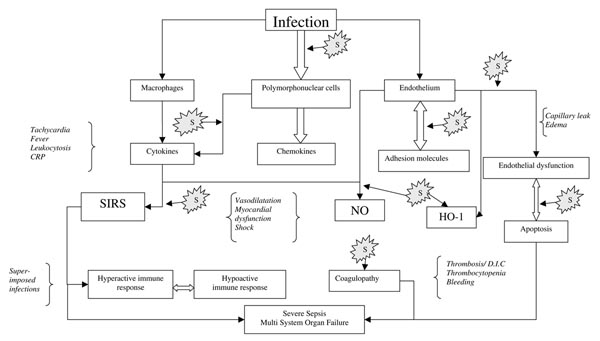
Key events leading from infection to multiorgan failure. For the sake of clarity, not all interactions and pathways are shown. 'S' denotes possible sites where statins might be exerting their beneficial effect. Shown in italics are some of the main clinical manifestations pertinent to specific elements of the inflammatory cascade. CRP, C-reactive protein; DIC, disseminated intravascular coagulation; HO, heme oxygenase; NO, nitric oxide; SIRS, systemic inflammatory response syndrome.
Microbial products recognized by phagocytic leukocytes and other immune cells form the molecular basis for the beginning of the sepsis syndrome. This process is accomplished by a variety of receptors that identify pathogen-associated conserved motifs. There are data suggesting that statins may interfere with this receptor–ligand interaction, thus blunting the first step in the activation of the cellular cascade [6].
The acute phase response includes a wide variety of mediators such as C-reactive protein, cytokines, and others. Their precise role in response to infection has not been fully elucidated. Extrapolating primarily from studies conducted in atherosclerosis, it is well known that statins markedly decrease these inflammatory markers [7]. Blunting this mal-adaptive exaggerated inflammatory response may be beneficial in sepsis.
Numerous studies using different models have demonstrated convincingly that statin pretreatment improves endothelial dysfunction, blunts apoptosis, and decreases levels of pro-inflammatory cytokines, chemokines, and adhesion molecules. The antithrombotic effects of statins may also ameliorate sepsis-induced coagulopathy [5,8,9]. Another novel pathway may play an important role in attenuating sepsis-induced endothelial dysfunction. Heme oxygenase (HO)-1 is an inducible, heat shock cytoprotective protein. Simvastatin activates and increases HO-1 in a concentration-dependent and time-dependent manner. This induction was observed in vascular smooth muscle and endothelial cells both in vitro and in vivo, suggesting that the anti-inflammatory, anti-proliferative, and antioxidant effects of simvastatin occur largely through induction of HO-1 [10,11].
An increase in nitric oxide (NO) production contributes to the hypotension and resistance to vasopressor therapy that occur in sepsis. Ample data using various models indicate that statins profoundly affect NO availability [5,12-14]. Specifically, in a rat pretreatment model simvastatin decreased NO overproduction and reverted the impaired vascular responsiveness induced by endotoxic shock [14]. Moreover, vascular hyporeactivity and peripheral vasodilatation are central characteristics of severe sepsis. In a randomized, placebo-controlled study in healthy volunteers challenged by lipopolysaccharide-induced inflammation, simvastatin exhibited potent vasoprotective properties [15].
An additional key characteristic of the hemodynamic perturbation in sepsis is myocardial dysfunction. In a pivotal report [16], mice pretreated with simvastatin and rendered septic by cecal ligation and perforation were found to exhibit a mean survival time close to four times that in untreated control animals. Complete preservation of cardiac function and hemodynamic status was observed [16]. Furthermore, in a similar study [17], in which treatment with various statins or placebo was initiated 6 hours after sepsis induction, when profound hemodynamic alterations were already evident, survival time was again significantly extended in treated animals and hemodynamic status was markedly improved. The importance of this latter report is further highlighted by its being the first to propose that statins may be beneficial as a therapeutic modality after the onset of sepsis-induced organ dysfunction.
Data in humans are lacking. In a prospective observational cohort study [18] it was found that prior statin therapy is associated with a decreased rate of severe sepsis and intensive care unit admission in patients admitted with acute bacterial infections. Even though this observational study was not powered to detect differences in sepsis-related mortality, a trend toward reduction in mortality was observed. In another large, community-based prospective study in which 11,362 patients were followed for up to 3 years (Almog and coworkers, unpublished data), we observed that therapy with statins may be associated with a reduced rate of infection-related mortality. This protective effect was independent of comorbidities and dissipated when the medication was discontinued.
There is growing interest among clinicians in the role that statins may play in preventing and treating serious infections. If such an effect of statins can be supported by randomized controlled clinical trials, then the implications could be far reaching. The stage is set for trials that will determine the precise role, if any, of statins in the primary prevention and treatment of this lethal yet potentially reversible syndrome.
Abbreviations
HO = heme oxygenase; NO = nitric oxide.
Competing interests
The author(s) declare that they have no competing interests.
Contributor Information
Victor Novack, Email: novack@bgu.ac.il.
Marius Terblanche, Email: Marius.Terblanche@sw.ca.
Yaniv Almog, Email: almogya@bgu.ac.il.
References
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9:517–524. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- Osler W. The Evolution of Modern Medicine, 1904 Reprinted in 2005. Whitefish, MT: Kessinger Publishing; 2005. [Google Scholar]
- Almog Y. Statins, inflammation, and sepsis: hypothesis. Chest. 2003;124:740–743. doi: 10.1378/chest.124.2.740. [DOI] [PubMed] [Google Scholar]
- Blanco-Colio LM, Tunon J, Martin-Ventura JL, Egido J. Anti-inflammatory and immunomodulatory effects of statins. Kidney Int. 2003;63:12–23. doi: 10.1046/j.1523-1755.2003.00744.x. [DOI] [PubMed] [Google Scholar]
- Weitz-Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, Bruns C, Cottens S, Takada Y, Hommel U. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;7:687–692. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, Gotto AM., Jr Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–1965. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- Steiner S, Speidl WS, Pleiner J, Seidinger D, Zorn G, Kaun C, Wojta J, Huber K, Minar E, Wolzt M, et al. Simvastatin blunts endotoxin-induced tissue factor in vivo. Circulation. 2005;111:1841–1846. doi: 10.1161/01.CIR.0000158665.27783.0C. [DOI] [PubMed] [Google Scholar]
- Dangas G, Badimon JJ, Smith DA, Unger AH, Levine D, Shao JH, Meraj P, Fier C, Fallon JT, Ambrose JA. Pravastatin therapy in hyperlipidemia: effects on thrombus formation and the systemic hemostatic profile. J Am Coll Cardiol. 1999;33:1294–1304. doi: 10.1016/S0735-1097(99)00018-2. [DOI] [PubMed] [Google Scholar]
- Lee TS, Chang CC, Zhu Y, Shyy JY. Simvastatin induces heme oxygenase-1: a novel mechanism of vessel protection. Circulation. 2004;110:1296–1302. doi: 10.1161/01.CIR.0000140694.67251.9C. [DOI] [PubMed] [Google Scholar]
- Grosser N, Hemmerle A, Berndt G, Erdmann K, Hinkelmann U, Schurgerc S, Wijayanti N, Immenschuh S, Schroder H. The antioxidant defense protein heme oxygenase 1 is a novel target for statins in endothelial cells. Free Radic Biol Med. 2004;37:2064–2071. doi: 10.1016/j.freeradbiomed.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Laufs U, Endres M, Custodis F, Gertz K, Nickenig G, Liao JK, Bohm M. Suppression of endothelial nitric oxide production after withdrawal of statin treatment is mediated by negative feedback regulation of rho GTPase gene transcription. Circulation. 2000;102:3104–3110. doi: 10.1161/01.cir.102.25.3104. [DOI] [PubMed] [Google Scholar]
- Wagner AH, Schwabe O, Hecker M. Atorvastatin inhibition of cytokine-inducible nitric oxide synthase expression in native endothelial cells in situ. Br J Pharmacol. 2002;136:143–149. doi: 10.1038/sj.bjp.0704678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giusti-Paiva A, Martinez MR, Felix JV, da Rocha MJ, Carnio EC, Elias LL, Antunes-Rodrigues J. Simvastatin decreases nitric oxide overproduction and reverts the impaired vascular responsiveness induced by endotoxic shock in rats. Shock. 2004:271–275. doi: 10.1097/10.shk.0000115756.74059.ce. [DOI] [PubMed] [Google Scholar]
- Pleiner J, Schaller G, Mittermayer F, Zorn S, Marsik C, Polterauer S, Kapiotis S, Wolzt M. Simvastatin prevents vascular hyporeactivity during inflammation. Circulation. 2004;110:3349–3354. doi: 10.1161/01.CIR.0000147774.90396.ED. [DOI] [PubMed] [Google Scholar]
- Merx MW, Liehn EA, Janssens U, Lutticken R, Schrader J, Hanrath P, Weber C. HMG-CoA reductase inhibitor simvastatin profoundly improves survival in a murine model of sepsis. Circulation. 2004;109:2560–2565. doi: 10.1161/01.CIR.0000129774.09737.5B. [DOI] [PubMed] [Google Scholar]
- Merx M, Liehn E, Graf J, van de Sandt A, Schaltenbrand M, Schrader J, Hanrath P, Weber C. Statin treatment after onset of sepsis in a murine model improves survival. Circulation. 2005;112:117–124. doi: 10.1161/CIRCULATIONAHA.104.502195. [DOI] [PubMed] [Google Scholar]
- Almog Y, Shefer A, Novack V, Maimon N, Barski L, Eizinger M, Friger M, Zeller L, Danon A. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation. 2004;110:880–885. doi: 10.1161/01.CIR.0000138932.17956.F1. [DOI] [PubMed] [Google Scholar]


