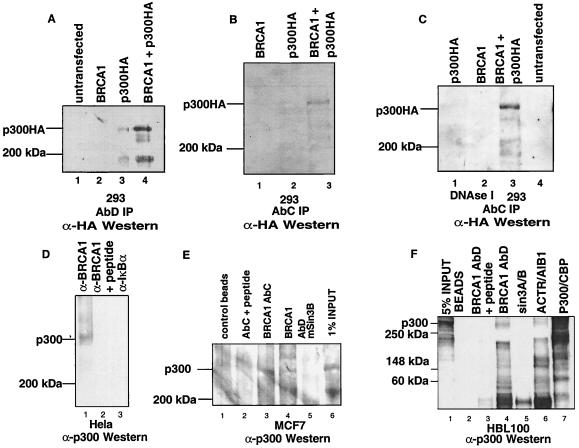
Figure 2.
(A) Immunoprecipitation with anti-BRCA1-AbD antibody of overexpressed BRCA1 and p300-HA in transiently transfected 293 cells. Transfected plasmids are indicated. Immune complexes were resolved by SDS/PAGE and immunoblotted with the 12CA5 anti-HA mAb. (B) Immunoprecipitation is as in A except that the immunoprecipitating antibody is AbC. The same immunoprecipitations were performed in the presence of 1,360 units/ml of DNaseI (C). Immunoprecipitation of endogenous BRCA1/p300 complexes from HeLa cells (D). Cell extracts from three 15-cm dishes were immunoprecipitated with anti-BRCA1 AbC antibody (lane 1), with the same antibody after preincubation with its cognate competitor “C” peptide (lane 2) or an unrelated rabbit antiserum (anti-IκBα, lane 3). Immunoprecipitated proteins were resolved by SDS/PAGE and immunoblotted with the C-20 anti-p300 antibody (Santa Cruz Biotechnology). Immunoprecipitations of one 15-cm dish per immunoprecipitation of MCF-7 breast cancer cells were done similarly (E). Immune complexes were precipitated by using either anti-BRCA1 AbC (lane 3) or AbD (lane 4). Negative controls were either precipitates with beads alone (lane 1), the immunoprecipitating antibody with prior incubation with its cognate peptide (lane 2), or the use of an unrelated antibody (Sin3B) (lane 5). One confluent 15-cm dish of HBL100 normal breast epithelial cells was used per immunoprecipitation reaction (F). The immunoprecipitating antibody is as noted above each lane. ACTR/AIB1 serves as a positive control (lane 6). The total amount of p300 signal in the extracts is given by the p300 immunoprecipitation reaction (lane 7). Sin3A and Sin3B antibodies were used as negative controls.