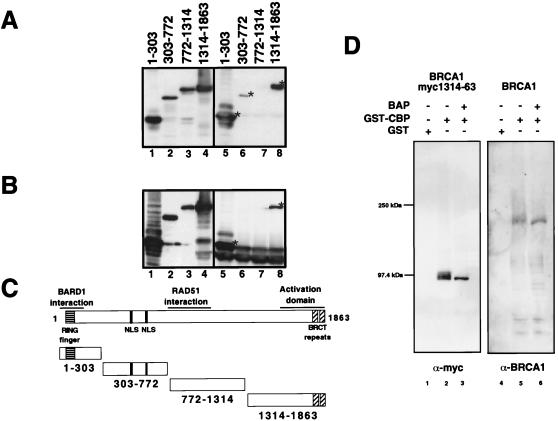
Figure 5.
(A) Mapping of the CBP interacting region on BRCA1 using GST-CBP 451–721 and myc-tagged BRCA1 fragments. Lanes 1–4 show anti-myc Western blots of the input cell extracts, and lanes 5–8 show the respective GST pulldowns. (B) Mapping by coimmunoprecipitation of cotransfected myc-tagged BRCA1 fragments and p300-HA using anti-HA antibody followed by anti-myc Western blotting. Input is shown in lanes 1–4 and immunoprecipitations in lanes 5–8. (C) Schematic structures of the overexpressed myc-tagged BRCA1 proteins. (D) Binding of BRCA1 to CBP is not affected by phosphorylation. The CBP/p300 interacting fragment of BRCA1 as well as full-length BRCA1 were overexpressed by transient transfection in 293T cells. The overexpressed proteins were immunoprecipitated with anti-BRCA1-AbD antibody and eluted off the antibodies. The proteins then were selectively dephosphorylated with bacterial alkaline phosphatase (BAP). Dephosphorylated and nondephosphorylated fragments then were assayed for binding to GST or GST-CBP 451–721. Bound proteins were resolved by SDS/PAGE and visualized by immunoblotting with either anti-myc or anti-BRCA1 AbD.