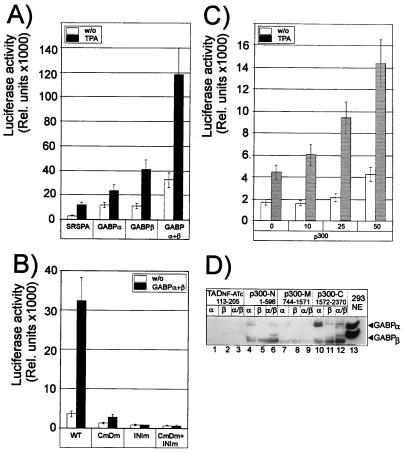
Figure 5.
GABPα and -β stimulate, in concert with p300, the activity of IL-16 promoter. (A) GABPα and -β stimulate the IL-16 promoter. Jurkat cells were cotransfected with a luciferase reporter gene controlled by the IL-16 promoter (from −408 to +88), SRSPA-based vectors expressing GABPα or -β, or both, as indicated. The cells were left untreated or stimulated with TPA (10 ng/ml) for 24 h. (B) The GABP-binding sites within the IL-16 promoter are of crucial importance for its activity. Human 293 cells were transfected with a wild-type IL-16 promoter/luciferase gene construct or mutated IL-16 promoter/luciferase constructs alone or together with vectors expressing GABPα and -β. CmDm, mutations in the ERE-C and ERE-D motifs (see Fig. 4B); INIm, mutations within the +12 GABP site which overlaps the transcriptional initiation site. (C) CBP/p300 enhance the activity of the IL-16 promoter in a dose-dependent manner. 293 cells were cotransfected with a luciferase reporter gene controlled by the IL-16 promoter (from −408 to +88) and 0–50 ng of a p300 expression vector as indicated. (D) Interaction between GABPα and p300. Equal amounts of bacterially expressed glutathione S-transferase/p300 fusion proteins spanning N-terminal, middle, or C-terminal portions of p300 immobilized on glutathione agrose beads were incubated with 500 ng of bacterially expressed GABPα, -β or -α+β. The bound proteins were eluted and immunodetected by using a mix of GABPα+β-specific Abs. As a control for p300/GABP interaction, a glutathione S-transferase fusion protein containing the N-terminal transacting domain of NF-ATc (TADNF-ATc, 113–205) was used. In lane 13, 50 μg of nuclear proteins from 293 cells were fractionated. The appearance of a weak GABPα band in lane 11 is the result of an accidental contamination of the GABPβ protein preparation with GABPα. The appearance of a second GABPα band in many lanes is probably a GABPα cleavage product. The lowest strong band is most likely the result of the crossreactivity of GABP Abs with a bacterial protein binding unspecifically to p300.