Figure 1.
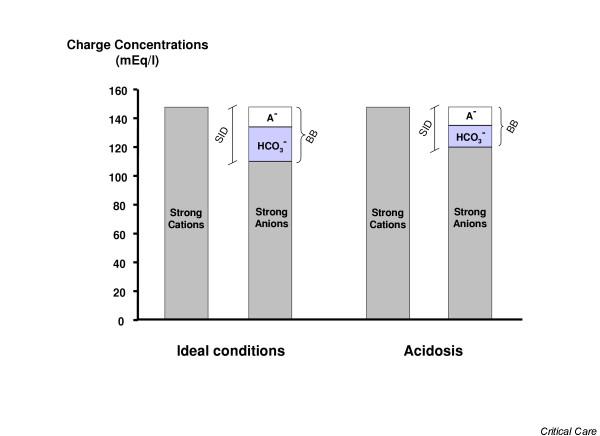
Gamblegram. The figure shows gamblegrams during ideal conditions and during acidosis. In ideal conditions the difference between positively and negatively charged strong ions is equal to 42 mEq/l (the strong ion difference [SID]) and, according to the law of electro-neutrality, is equivalent to the buffer base (BB; i.e. the sum of [HCO3-], [A-] and [H2PO4-], where A- are the weak acids in dissociated form, mainly albumin). During acidosis, SID decreases but the law of electro-neutrality is still satisfied. It follows that base excess = BBactual - BBideal = SIDacidosis - SIDideal.
