Figure 2.
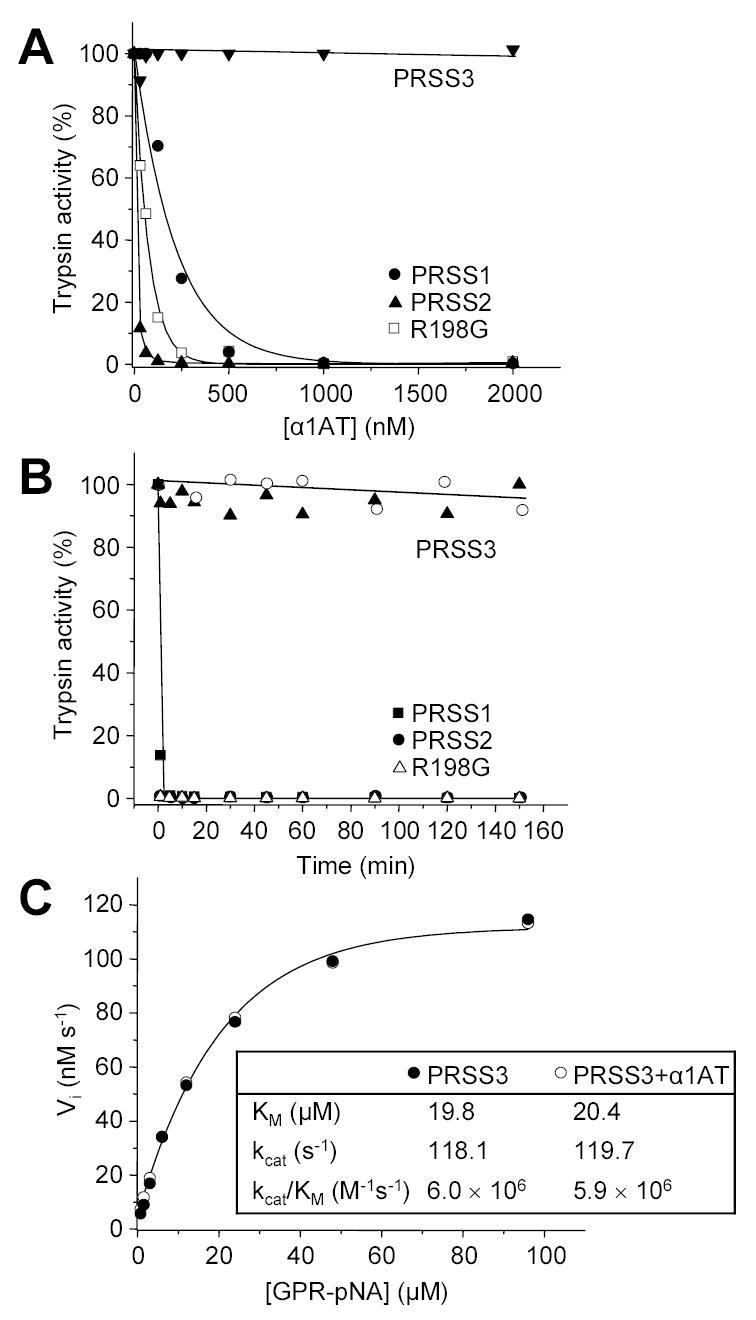
Inhibition of human trypsins by α1-antitrypsin (α1AT). A. Cationic trypsin (PRSS1), anionic trypsin (PRSS2), mesotrypsin (PRSS3) and the R198G mesotrypsin mutant were incubated at 20 nM concentration with the indicated concentrations of α1AT in 100 μL final volume of 0.1 M Tris-HCl (pH 8.0) and 1 mM CaCl2, at room temperature for 20 min. Trypsin activity was then assayed with 0.1 mM N-CBZ-Gly-Pro-Arg-p-nitroanilide (final concentration), and expressed as percentage of initial activity (without inhibition). B. Trypsins (2 μM concentration) were incubated with 5 μM α1AT (final concentration) in 0.1 M Tris-HCl (pH 8.0), 2 mg/mL BSA, and 1 mM CaCl2 at 37 C°. At indicated time-points 2 μL aliquots were withdrawn and trypsin activity was measured. Recombinant α1AT was used to inhibit trypsins in these experiments, with the exception of mesotrypsin (PRSS3), which was incubated with recombinant (solid triangles) and native α1AT purified from human serum (open circles). C. Competitive inhibition of mesotrypsin by α1AT. The initial rate (Vi) of substrate hydrolysis by 1 nM mesotrypsin (final concentration) was measured at the indicated N-CBZ-Gly-Pro-Arg-p-nitroanilide (GPR-pNA) concentrations, in the presence (○) or absence (●) of 7.5 μM α1AT (final concentration), in 0.1 M Tris-HCl (pH 8.0) and 1 mM CaCl2 at room temperature. The KM and kcat parameters were determined from hyperbolic fits.
