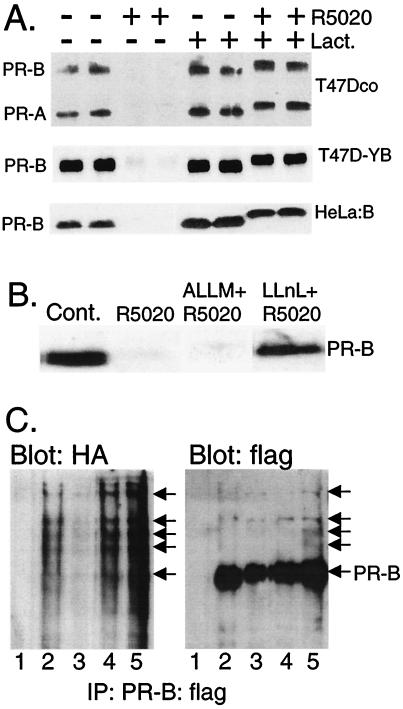
Figure 1.
PR down-regulation is mediated by the 26S proteasome. (A) T47Dco breast cancer cells, which express the natural B and A isoforms of PRs constitutively (15), and T47D-YB (16) and HeLa:B cells (17), which stably express the recombinant B isoform of PRs, were treated without or with the progestin R5020 (10 nM) for 12 h in the absence (DMSO solvent) or presence of lactacystin [Lact. (10 μM)], and PR protein (100 μg of total protein per lane) was detected by immunoblotting with PR-specific monoclonal antibodies. (B) Inhibition of PR down-regulation by calpain inhibitor I, but not calpain inhibitor II. T47D-YB cells were treated as in A, except with calpain inhibitor II [ALLM (25 μM)] or calpain inhibitor I [LLnL (25 μM)]. Each compound alone had no effect on PR abundance. (C) PR-ubiquitin conjugates in cells transiently overexpressing ubiquitin and PR-B. HeLa cells were transiently cotransfected with expression vectors encoding HA-tagged ubiquitin and epitope-flag-tagged wild-type PR-B (PR-B:flag) and treated for 4 h without (lane 2) or with R5020 (lane 3; 10 nM) in the absence or presence of lactacystin (lane 4; 10 μM) or LLnL (lane 5; 25 μM). PRs were immunoprecipitated by using anti-flag M2 affinity gel and visualized by immunoblotting with either HA- or flag-specific antibodies. Lane 1, nonspecific antibody and similar affinity gel. High-molecular-weight ubiquitinated forms of PR-B:flag are indicated (arrows).