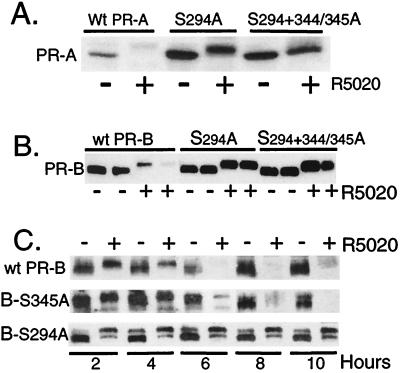
Figure 3.
Mutation of serine-294 to alanine at a MAPK consensus site stabilizes PR in the presence of progestin. (A) Mutant S294A and S294+S344/345A PR-As are resistant to R5020-induced receptor down-regulation. HeLa cells were transiently transfected with cDNA vectors (1 μg) encoding either wild-type PR-A or the S294A or S294+344/345A mutants of PR-A and then treated without or with R5020 (10 nM) for 8 h. PR protein was measured in whole-cell lysates (100 μg) by immunoblotting. (B) Mutant S294A and S294+344/345A PR-Bs are resistant to R5020-induced receptor down-regulation. Duplicate cultures of HeLa cells were transfected with wild-type PR-B or each phosphomutant of PR-B and treated without or with R5020 for 18 h. Protein (100 μg per lane) was loaded, and PR levels were measured by using PR-specific monoclonal antibodies. (C) The S294A PR-Bs stably expressed in T47D-Y cells are resistant to ligand-induced down-regulation. PR-negative breast cell lines stably expressing either an S344/345A (B-S345A) mutant or the S294A (B-S294A) mutant PR-B receptors were produced by transfection of receptor expression vectors containing the neomycin-resistance gene into T47D-Y cells and selected for growth in G418. Neoresistant clonal cell lines were screened for PR expression, and S344/345A or S294A PR-containing cells or wild-type PR-B-containing T47D-YB cells (wt-PR-B) were treated without or with R5020 (10 nM) for 2–10 h; protein levels were measured with PR-specific antibodies. 150–200 μg of protein was loaded per lane.