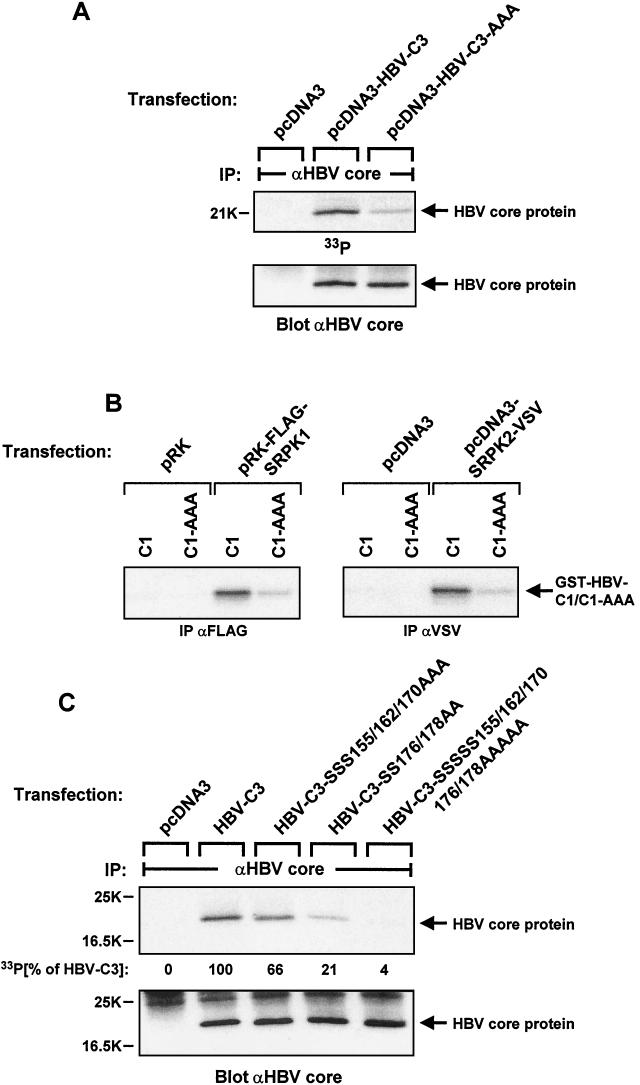
FIG.5.
Analysis of in vivo phosphorylation and SRPK-mediated in vitro phosphorylation of HBV core protein. (A) HuH-7 cells were transiently transfected in six-well dishes with 1.5 μg of either empty expression vector, plasmid encoding wild-type HBV core protein, or plasmid encoding mutant HBV core protein devoid of the three reported C-terminal serine phosphorylation sites (serines 155, 162, and 170) per well. On the second day after transfection, cells were labeled with 50 μCi of 33Pi per ml for 3.5 h. Lysates were then subjected to immunoprecipitation (IP) with anti-HBV core antibody. After gel electrophoresis and transfer onto a nitrocellulose membrane, HBV core protein phosphorylation was first visualized by autoradiography (upper panel), followed by immunoblotting of the same filter with anti-HBV core protein antibody (lower panel). (B) COS-7 cells were transiently transfected in six-well dishes with either control plasmids or expression constructs encoding FLAG-tagged SRPK1 or VSV-tagged SRPK2 (1.5 μg/well each). After cell lysis and immunoprecipitation (IP) with anti-FLAG or anti-VSV antibodies, in vitro kinase reactions were performed in the presence of [γ-32P]ATP and GST-HBV-C1 core fusion protein with (C1) or without the three previously mapped C-terminal serine phosphorylation sites (C1-AAA, serines 155, 162, and 170 mutated to alanines). After gel electrophoresis, phosphate incorporation into substrate protein was visualized by autoradiography. (C) HuH-7 cells were transiently transfected in six-well dishes with plasmids encoding either wild-type HBV core protein or its mutants lacking serine phosphorylation sites in the C-terminal part as indicated (1.5 μg/well each). After metabolic labeling with 50 μCi of 33Pi per ml for 3.5 h, cell lysates were processed as described for panel A. HBV core protein phosphorylation was visualized by autoradiography (upper panel) and quantified by a PhosphorImager, and the same filter was then probed with anti-HBV core protein antibody (lower panel).