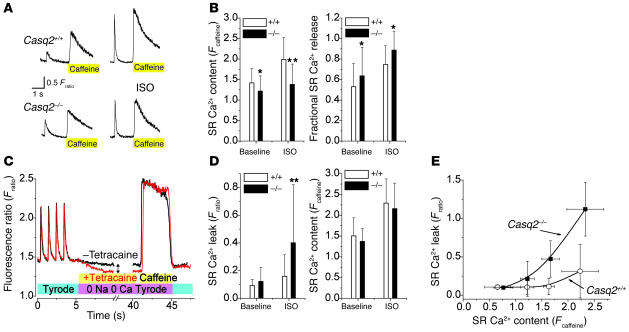
Figure 5. Casq2–/– myocytes have largely preserved SR Ca2+ release and SR Ca2+ content under basal conditions, but isoproterenol application causes increased SR Ca2+ leak.
(A) Representative examples of rapid application of caffeine (10 mmol/l) to a Casq2+/+ (top) and a Casq2–/– myocyte (bottom). Myocytes were field stimulated at 1 Hz to maintain consistent SR Ca2+ load. Note the increased twitch transient and caffeine-induced transients in the presence of ISO (1 μmol/l; right). The height of the caffeine-induced Ca2+ transient was used as a measure of total SR Ca2+ content (30). Fractional SR Ca2+ release was calculated by dividing the height of the last twitch transient by the height of the caffeine transient. (B) Comparison of average SR Ca2+ content (left) and fractional SR Ca2+ release (right). *P < 0.05, **P < 0.01. Casq2+/+ myocytes: n = 41 (baseline) and 27 (ISO); Casq2–/–myocytes: n = 70 (baseline) and 37 (ISO). (C) Protocol used to measure SR Ca2+ leak as described in ref. 32. Plasma membrane Ca2+ flux is eliminated by removal of extracellular Na+ and Ca2+. The drop in steady-state [Ca2+]i (double arrow) represents a shift of Ca2+ from the cytosol to the SR when RyR2 channels are inhibited by tetracaine (1 mmol/l) and was used as a measure of SR Ca2+ leak. (D) Comparison of average SR Ca2+ leak (left) and SR Ca2+ content in the presence of tetracaine (right). Note that when SR Ca2+ leak was blocked by tetracaine, SR Ca2+ content was not significantly different between the 2 groups. **P < 0.01. Casq2+/+ myocytes: n = 32 (baseline) and 45 (ISO); Casq2–/–myocytes: n = 29 (baseline) and 42 (ISO). (E) SR Ca2+ leak in the presence of ISO plotted as a function of SR Ca2+ content. Note that the SR Ca2+ leak of Casq2–/– myocytes remained SR load dependent but was shifted to the left compared with that of Casq2+/+ myocytes.