Figure 3. A model for IKK activation by TRAF6 ubiquitination.
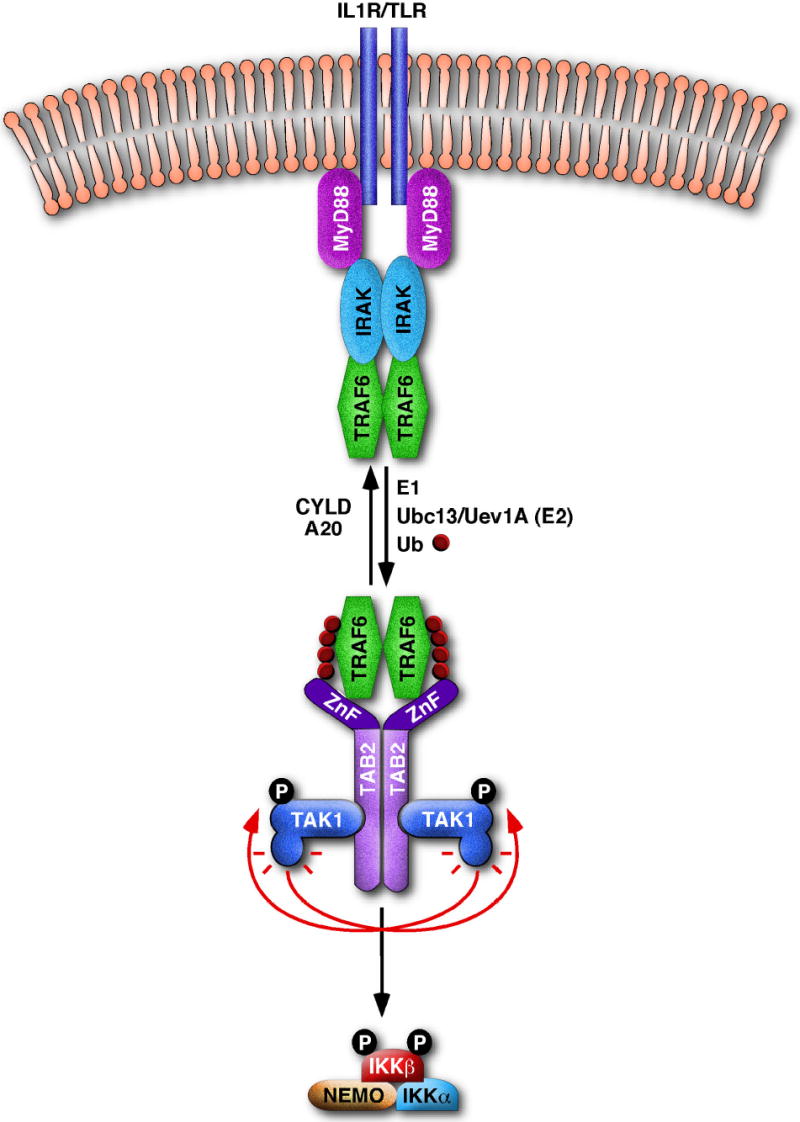
Stimulation of IL-1R or TLR leads to the recruitment of MyD88, IRAK and TRAF6 to the receptor complex. This may facilitate TRAF6 oligomerization and activate its ubiquitin ligase activity, leading to K63-linked polyubiquitination of targets including Nemo (not shown) and TRAF6 itself. This polyubiquitination reaction requires E1 and Ubc13/Uev1A, and can be reversed by deubiquitination enzymes such as CYLD and A20. Ubiquitinated TRAF6 is recruited to the TAK1/TAB2 complex through binding of K63-linked polyubiquitin chains to the NZF domain of TAB2 as well as by the direct interaction between TRAF6 and TAB2. This binding may facilitate the dimerization or oligomerization of the TAK1/TAB2 complex, promoting its autophosphorylation and TAK1 activation. TAK1 then phosphorylates IKKβ, resulting in its activation.
