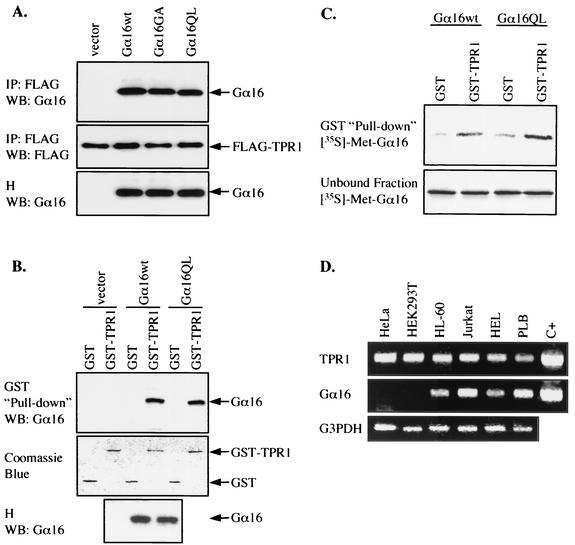
FIG. 1.
Interaction between Gα16 and TPR1. (A) HEK293T cells were transiently transfected with the FLAG-TPR1 construct either alone or with expression vectors for Gα16wt, Gα16GA (inactive mutant), and Gα16QL (active mutant). Whole-cell lysates were immunoprecipitated with an anti-FLAG agarose affinity gel. The immunoprecipitates (IP) and cell homogenates (H) were resolved by SDS-PAGE (10% polyacrylamide). Coimmunoprecipitation of Gα16 with FLAG-TPR1 was examined by Western blotting (WB) with anti-Gα16 serum (top panel). FLAG-TPR1 in the IP was detected with an anti-FLAG MAb and serves as a loading control (middle panel). The blot was probed for the expression of Gα16 in the cell homogenates (bottom panel). In the absence of FLAG-TPR1, no Gα16 is detectable in the IP fraction (not shown). (B) Cells were transfected with empty vector or with expression vectors for Gα16wt or Gα16QL. Whole-cell lysates were incubated with GST (negative control) or GST-TPR1 fusion protein immobilized on glutathione-Sepharose beads. Bound proteins were eluted and analyzed by Western blotting. The ability of Gα16 to interact with GST or GST-TPR1 was determined by blotting the eluate with anti-Gα16 (top panel). The expression of Gα16 in the homogenates was assessed as in panel A (bottom panel). GST and GST-TPR1 were detected by staining with Coomassie blue (middle panel) and served as loading controls. (C) Equivalent amounts of GST or GST-TPR1, immobilized on glutathione-Sepharose beads, were incubated with in vitro-translated [35S]methionine-labeled Gα16wt or Gα16QL. After the beads were pelleted, both the unbound proteins recovered from the supernatants (bottom panel) and the bound proteins (top panel) were subjected to SDS-PAGE and autoradiography. (D) Total RNA was isolated from the human cervical carcinoma cell line HeLa, kidney epithelial cell line HEK293T, promyelocytic cell line HL-60, leukemic T-cell line Jurkat, erythroleukemic cell line HEL, and myelomonoblastic cell line PLB 985. The expression of TPR1 (top panel) and Gα16 (middle panel) mRNAs was detected by reverse transcriptase PCR using specific primers. In parallel, 1 ng of DNA encoding TPR1 or Gα16 was used as a positive control (C+) for the PCR. Glyceraldehyde 3-phosphate dehydrogenase (G3PDH) expression serves as PCR and loading controls (bottom panel). All results presented are representative of at least three independent experiments.