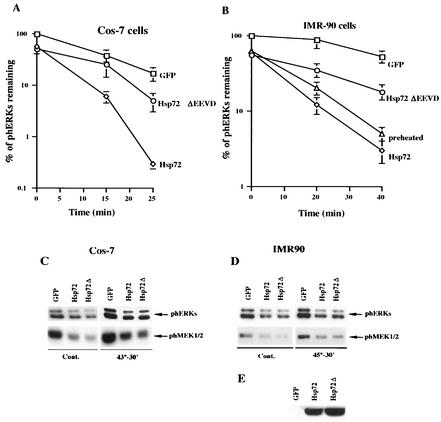
FIG. 7.
Hsp72 and Hsp72ΔEEVD suppress HS mediated activation of ERKs and MEK1/2. (A and B) Quantification of the rates of ERK dephosphorylation in cells overexpressing the normal or mutant form of Hsp72 or preheated at 45°C for 30 min. Cos-7 cells (A) or IMR90 cells (B) were infected with adenovirus expressing either GFP (□), Hsp72 (⋄), or Hsp72ΔEEVD (○). At 36 h postinfection, cells were heated for 30 min at 43°C (A) or 45°C (B), and the rate of ERK dephosphorylation was assayed. The percentage of ERK activity was normalized to GFP-expressing cells. Each curve represents the average of at least three experiments. Alternatively, in panel B IMR90 cells were subjected to HS at 45°C for 30 min, recovered at 37°C for 18 h, and subjected to a second HS at 45°C for 30 min (preheated). (C and D) Hsp72 and Hsp72ΔEEVD suppress background and HS-induced ERKs and MEK1/2 activity. Cos-7 cells (C) and IMR90 cells (D) were infected as described for panels A and B and either left untreated (Cont.) or heated at 43°C (A) or 45°C (B). The extent of ERK and MEK1/2 activation was assayed by immunoblotting cell lysates with anti-phospho-ERK or anti-phosph-MEK1/2 antibody. (E) Expression of Hsp72 or Hsp72ΔEEVD in IMR90 cells infected with the corresponding adenovirus. IMR90 cells were infected with GFP, Hsp72, or Hsp72ΔEEVD as described above. Cell lysates were immunoblotted with SPA820 antibody, which recognizes both normal and mutant Hsp72 proteins.