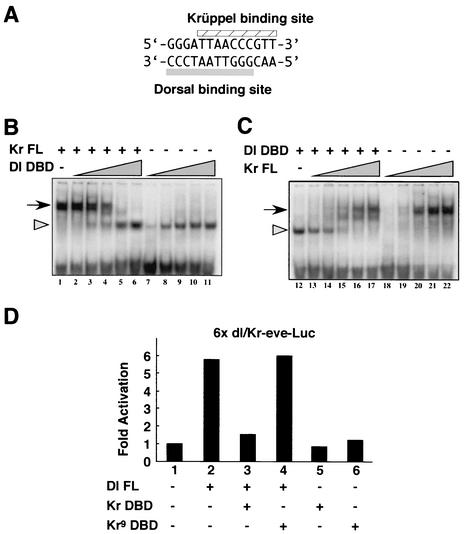
FIG. 4.
Krüppel and Dorsal proteins compete for overlapping binding sites. (A) Sequence of the synthetic 14-bp sequence containing overlapping Bicoid, Dorsal, and Krüppel binding sites. Bicoid recognizes the core sequence GGATTA. The Dorsal and Krüppel sites are indicated. (B and C) Gel shift assays. A 30-bp DNA fragment, containing the core 14-bp sequence shown in panel A plus 8 bp of linker sequence on either side was labeled with [γ-32P]ATP by T4 polynucleotide kinase. A His-tagged Krüppel protein (Kr FL) and a His-tagged Rel domain from Dorsal (Dl DBD) were used for the binding assays. Increasing amounts of Dl DBD protein with or without constant amounts of Kr FL protein (B) and the reciprocal condition (C) were used. After the binding, the resulting complexes were resolved by electrophoresis in nondenaturing 8% polyacrylamide gels. Arrows and arrowheads, Krüppel-probe and the Dorsal-probe complexes, respectively. Supershifted complexes were not observed, indicating that the two proteins did not bind to the probe at the same time. (D) Transient-transfection assays. Six tandem copies of the 14-bp synthetic sequence were attached to an eve-luciferase reporter gene containing the minimal eve promoter. Addition of a Dorsal expression vector caused more than a fivefold induction in expression above background levels (compare lanes 1 and 2). However, this induction was lost upon coexpression of the Krüppel DNA-binding domain (Kr DBD; lane 3). The Kr DBD lacks both the N-terminal and C-terminal repression domains, including the optimal dCtBP interaction motif at positions 464 to 470 of the wild-type protein. Expression of a mutant form of the Kr DBD that contains a single amino acid substitution in one of the C2H2 zinc fingers fails to repress activation by Dorsal (Kr9 DBD; lane 4). The mutant peptide is unable to bind DNA (25).