Table 1.
Synthesis of Syn N-Donor Ligands 5a–i
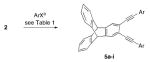
| entry | ArXb-d | product | yield (%) (overall yield)e |
|---|---|---|---|
| 1 |
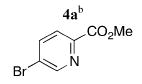
|
5a | 93 (47)f |
| 2 |
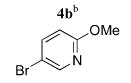
|
5b | 79 (40)f |
| 3 |
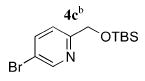
|
5c | 97 (40)f |
| 4 |
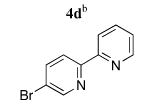
|
5d | 93 (49)f |
| 5 |
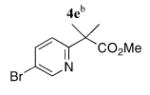
|
5e | 80 (47)d |
| 6 |
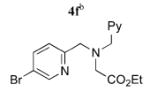
|
5f | 99 (50)f,g |
| 7 |
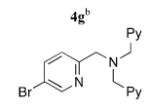
|
5g | 87 (44)f,g |
| 8 |
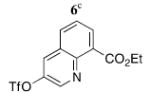
|
5h | 67 (9)h |
| 9 |
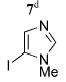
|
5i | 63 (32)f |
2.2–2.5 equiv of ArX was used.
10 mol % of Pd(PPh3)4, Et3N, THF, 55 °C.
10 mol % of PdCl2(PPh3)2, 5 mol % of CuI, Et3N, THF, rt.
10 mol % of PdCl2(PPh3)2, piperidine, 65 °C.
The overall yield is calculated for the longest linear sequence from commercially available materials.
Overall yield from 1,2,4,5-tetrabromobenzene.
The yield was measured by 1H NMR spectroscopy using an internal standard.
Overall yield from anthranilic acid.
