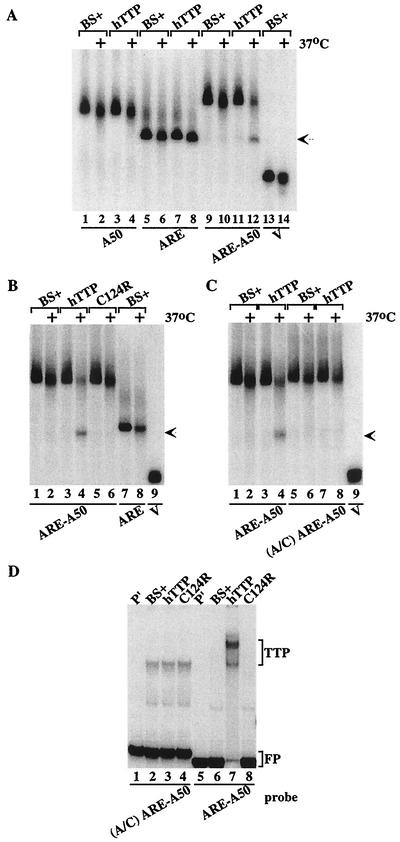
FIG. 2.
Cell-free deadenylation of polyadenylated, ARE-containing RNA probes. In panels A to C, 293 cell extracts were incubated with 32P-labeled RNA probes on ice (no symbol) or at 37°C (+) for 60 min, and EDTA (final concentration, 20 mM) was added to stop the reaction. RNA was then isolated and subjected to electrophoresis on urea-polyacrylamide gels, followed by autoradiography. The arrow in panels A to C indicates the migration position of the ARE probe and the deadenylated product of probe ARE-A50. (A) Incubation of the RNA probes A50 (lanes 1 to 4), ARE (lanes 5 to 8), ARE-A50 (lanes 9 to 12), and V (lanes 13 and 14) with extracts from 293 cells transfected with vector alone (BS+) or CMV.hTTP.tag (hTTP). (B) Incubation of the RNA probes ARE-A50 (lanes 1 to 6) and ARE (lanes 7 and 8) with extracts from 293 cells transfected with vector alone (BS+), CMV.hTTP.tag (hTTP), or the TTP zinc finger mutant (C124R). The position of probe V migration is shown in lane 9. (C) Incubation of the RNA probe ARE-A50 (lanes 1 to 4) and the mutant probe (A/C) ARE-A50 (lanes 5 to 8) with extracts from 293 cells transfected with vector alone (BS+) or CMV.hTTP.tag (hTTP). The position of probe V migration is shown in lane 9. (D) An electrophoretic mobility shift assay was performed by using extracts from 293 cells transfected with vector alone (BS+), CMV.hTTP.tag (hTTP), or the TTP zinc finger mutant (C124R) with probes (A/C) ARE-A50 (lanes 2 to 4) or ARE-A50 (lanes 6 to 8). The final reaction products were separated on an 8% nondenaturing polyacrylamide gel followed by autoradiography. Lanes 1 and 5 (P′) were loaded with probe alone (RNase T1 digested). The TTP-RNA complexes formed (TTP) and the migration position of the free probe (FP) are indicated.