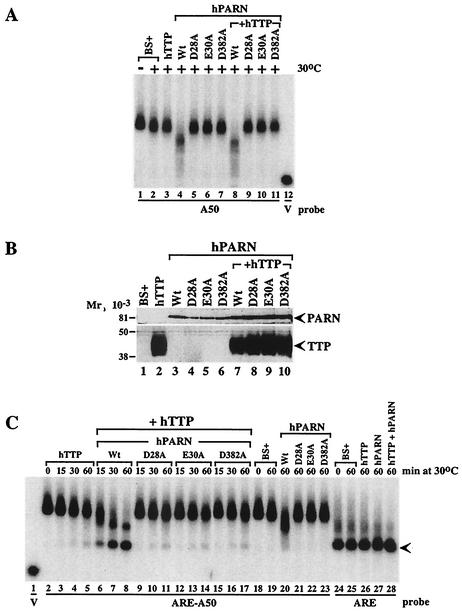
FIG. 9.
Effect of inactive PARN on probe deadenylation in the presence or absence of TTP. Extracts (5 μg of protein) from 293 cells transfected with vector alone (BS+), CMV.hTTP.tag (hTTP), CMV.hPARN.flag (hPARN), or its mutants (D28A, E30A, and D382A), or CMV.hTTP.tag together with CMV.hPARN.flag (or its mutants) were incubated with probes ARE-A50, ARE, or A50, as indicated. Deadenylation assays were carried out at 30°C in this experiment to slow the reaction rate. The buffer used in these experiments contained 100 mM KCl, 1 mM MgCl2, and 10 mM HEPES (pH 7.6).(A) Effect of native PARN (Wt) and the three mutant PARN proteins on the deadenylation of the poly(A) probe (A50) in the presence or absence of TTP, as indicated, either at 0°C (−) or after 60 min at 30°C (+). BS+ refers to extracts from cells transfected with vector alone. Probe V is the remnant vector sequence with no attached poly(A) tail. (B) Western blot demonstrating the expression of native and mutant PARN species in these experiments, as indicated. The symbols are the same as in panel A. The expression of FLAG-tagged TTP is also indicated by a Western blot with the FLAG antibody. The positions of molecular weight standards are shown on the left. (C) Effect of the extracts from vector alone (BS+), TTP alone, native and mutant PARN alone, and various combinations on the deadenylation of ARE-containing, polyadenylated probe ARE-A50, as well as the nonpolyadenylated ARE-containing probe ARE. The times of incubation at 30°C are indicated; the position of the completely deadenylated probe is indicated by the arrow. See the text for further details.