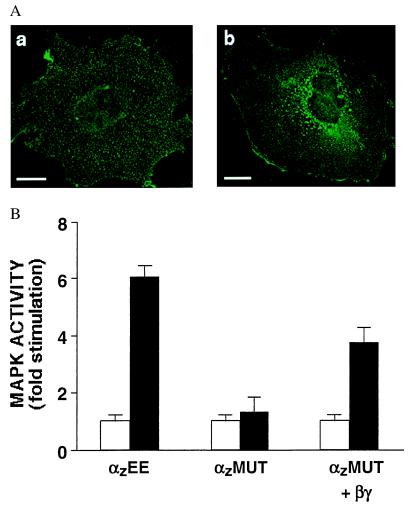
Figure 1.
Alanine mutations at a βγ-binding interface of αz cause the mutant protein, αzMUT, to mislocalize to intracellular membranes. (A) Immunofluorescence microscopy of CHO cells transfected with wild-type αz (a) or αzMUT (b). Although the fluorescent signal for wild-type αz (a) is not confined to the outer perimeter of the cell, the staining pattern indicates localization at the PM. This pattern is due to the extremely flat and thin shape of CHO cells under our culture conditions; using confocal microscopy, we previously observed the same pattern of fluorescence for PM-localized proteins in CHO cells (1). (Bar = 20 μm.) (B) MAPK assays were performed on CHO cells transfected 48 h earlier with the D2 dopamine receptor and HA-MAPK, together with wild-type αz, αzMUT, or αzMUT plus β1 and wild-type γ2. MAPK activity was determined after a 4-h treatment with pertussis toxin (100 ng/ml) and a 7-min exposure to the D2 agonist (10 μM quinpirole, black bars) or no agonist (white bars). Data shown are the means ±2 SEM of four experiments.