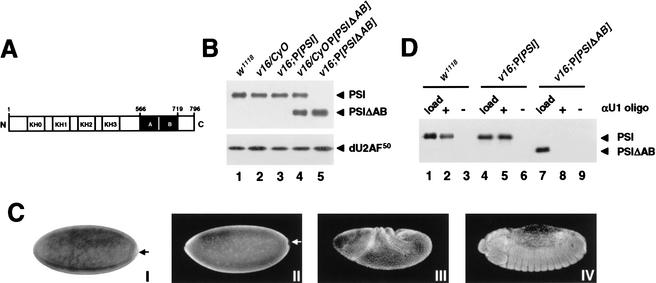
Figure 3.
Analysis of PSI and PSIΔAB transgene products. (A) Schematic representation of the PSI domain structures. The KH-type RNA-binding domains (KH0–KH3) and the protein–protein interaction motifs (repeats A and B) are boxed in white and black, respectively. The PSIΔAB protein lacks amino acids 566–719. (B) Immunoblot analyses of whole-fly protein extracts prepared from w1118 males (lane 1) or transgenic males balanced (lanes 2,4) or homozygous for the v16 mutation (lanes 3,5) and carrying a PSI (lane 3) or PSIΔAB (lanes 4,5) cDNA transgene were performed as in Figure 1B. (C) Stage 4 w1118 (I,II), stage 8 v16;P[PSI] (III), or stage 14 v16;P[PSIΔAB] (IV) embryos were fixed and hybridized with a digoxygenin-labeled anti-sense PSI RNA probe (I) or stained with affinity-purified anti-PSI polyclonal antibodies and Alexa Fluor secondary antibodies (II–IV). No staining was detected in the pole cells (arrows). Identical patterns of expression were observed in w1118, v16;P[PSI], and v16;P[PSIΔAB] embryos. (D) PSI–U1 snRNP interaction in embryonic nuclear extracts. Embryonic nuclear extracts, prepared using 0–12-h w1118 (lanes 1,2), v16;P[PSI] (lanes 3,4), or v16;P[PSIΔAB] (lanes 5,6) embryos were incubated in the absence (−) or presence (+) of a biotinylated 2‘-O-methyl anti-sense U1 snRNA oligonucleotide. U1 snRNP particles affinity-selected on streptavidin-agarose beads were resolved on a 10% SDS-polyacrylamide gel, transferred to nitrocellulose, and analyzed by immunoblot using affinity-purified anti-PSI polyclonal antibodies. To show that the level of PSI or PSIΔAB protein expression in rescued v16 embryos is equivalent to the normal level of PSI expression in w1118 embryos, 1/50 of the input nuclear extracts were analyzed in parallel (load; lanes 1,4,7).