Figure 5.
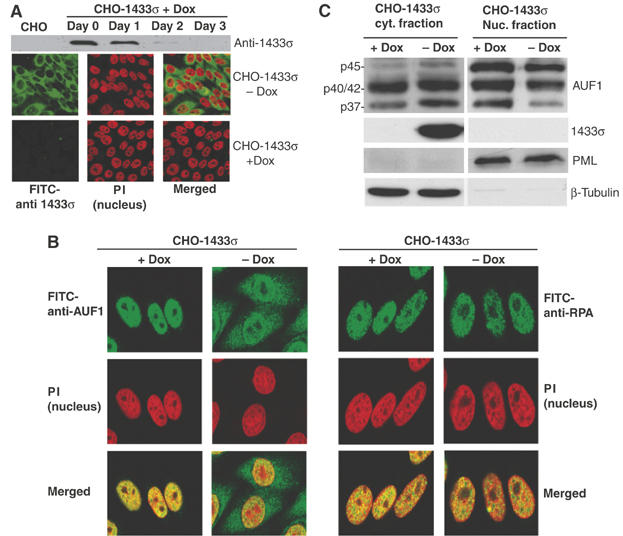
14-3-3σ expression promotes cytoplasmic accumulation of p37 AUF1. (A) A stable CHO cell line expressing 14-3-3σ under the control of a doxycycline (Dox)-suppressed promoter (pBSTR1-14-3-3σ) was developed. (Upper panel) Cells treated with Dox for up to 3 days to inhibit 14-3-3 expression were lysed and examined for 14-3-3σ protein levels by immunoblot analysis with 14-3-3σ antibody. (Lower panels) Cells were treated with Dox or mock treated for 3 days and examined by immunofluorescence microscopy with 14-3-3σ antibody followed by FITC-conjugated secondary antibody. Cell nuclei were visualized by staining with propidium iodide (PI). Representative individual panels of cells and panels with merged P.I./FITC staining are shown. (B) (Left panels) 14-3-3σ cells treated with Dox (+Dox) or mock treated (−Dox) under the same conditions as above, were fixed and stained for endogenous AUF1 with anti-AUF1 antibody followed by FITC-conjugated secondary antibody. Nuclei were visualized with PI. (Right panels) As a control, nuclear protein RPA, was stained with RPA antibody followed by FITC-conjugated secondary antibody. Representative panels of cells from at least three independent analyses are shown. (C) CHO-14-3-3σ cells were treated with Dox for 3 days or mock treated, collected and fractionated into cytoplasmic and nuclear fractions. Proteins were resolved by SDS–PAGE and detected by immunoblot analysis with AUF1 antibody and ECL. β-Microtubulin, a cytoplasmic protein, and PML, a nuclear protein, were used as controls for equal loading and fractionation efficacy. Representative results of at least three independent experiments are shown.
