Abstract
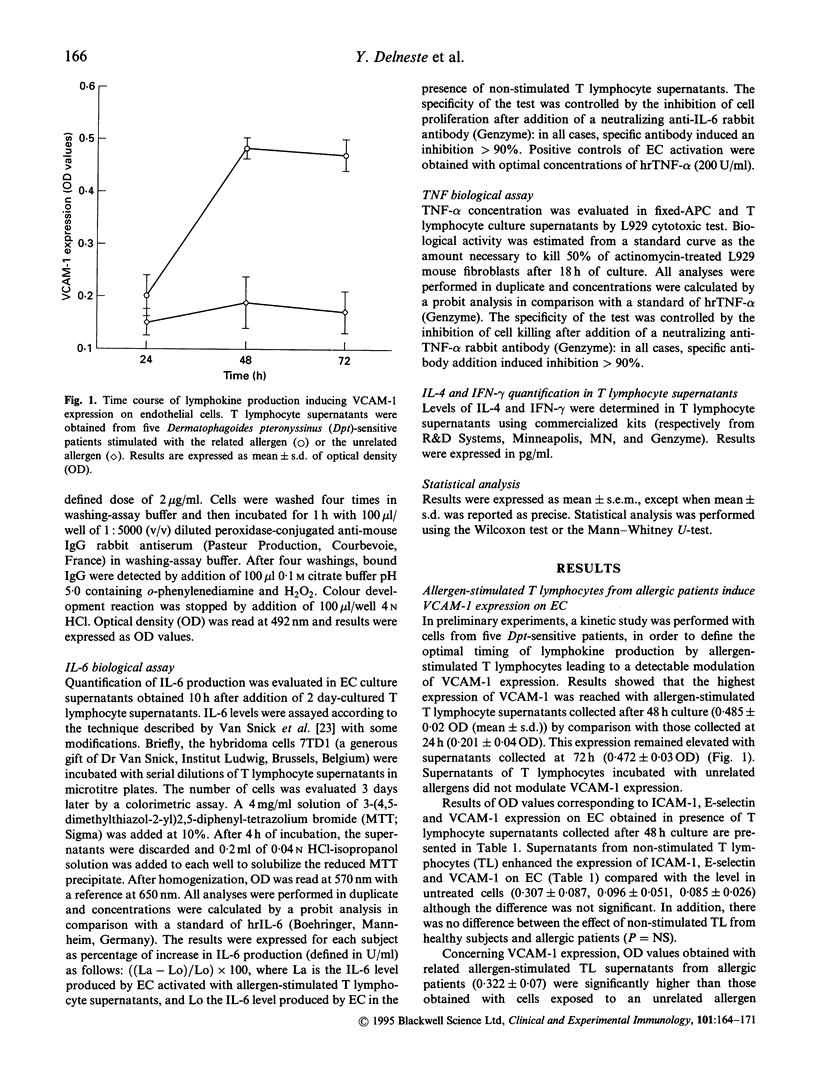
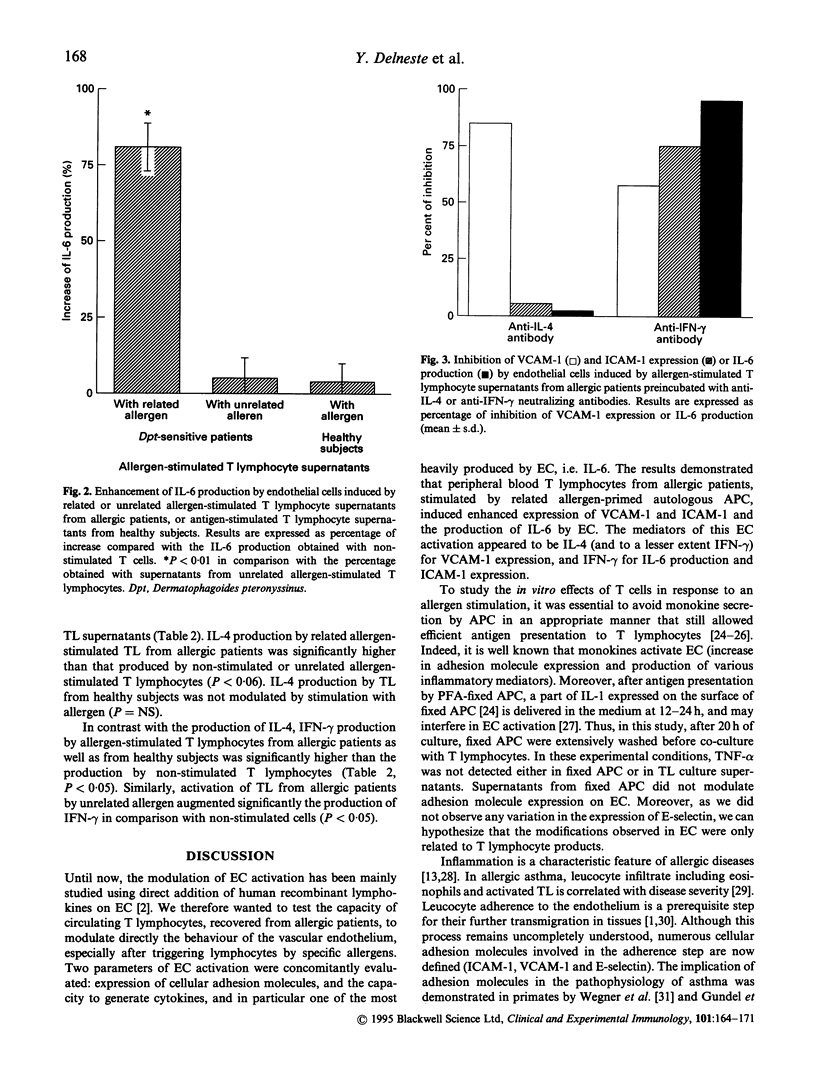
Adhesion of inflammatory cells to endothelium is a critical step for their transvascular migration to inflammatory sites. To evaluate the relationship between T lymphocytes (TL) and vascular endothelium, supernatants from allergen-stimulated TL obtained from patients sensitive to Dermatophagoides pteronyssinus (Dpt) versus healthy subjects were added to endothelial cell (EC) cultures. TL were stimulated by autologous-activated antigen-presenting cells (APC) previously fixed in paraformaldehyde to prevent monokine secretion. Two parameters were measured: the expression of adhesion molecule and the production of IL-6. Related allergen-stimulated TL supernatants from allergic patients induced an increase of VCAM-1 and intercellular adhesion molecule-1 (ICAM-1) expression when supernatants of the control groups (TL exposed to an unrelated allergen or not stimulated or TL obtained from healthy subjects) did not. E-selectin expression was not modulated whatever the supernatant added to EC culture. IL-6 production by EC was significantly enhanced after activation with related allergen-stimulated TL supernatants from allergics compared with control supernatants. Induction of VCAM-1 expression was inhibited by adding neutralizing antibodies against IL-4, whereas IL-6 production and ICAM-1 expression were inhibited by anti-interferon-gamma (IFN-gamma) antibodies. Enhanced production of IL-4 and IFN-gamma was detected in related allergen-stimulated TL supernatants from allergic subjects compared with the different supernatants. These data suggest that allergen-specific TL present in the peripheral blood of allergic patients are of Th1 and Th2 subtypes. Their stimulation in allergic patients may lead to the activation of endothelial cells and thereby participate in leucocyte recruitment towards the inflammatory site.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzawi M., Bradley B., Jeffery P. K., Frew A. J., Wardlaw A. J., Knowles G., Assoufi B., Collins J. V., Durham S., Kay A. B. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am Rev Respir Dis. 1990 Dec;142(6 Pt 1):1407–1413. doi: 10.1164/ajrccm/142.6_Pt_1.1407. [DOI] [PubMed] [Google Scholar]
- BOYUM A. SEPARATION OF WHITE BLOOD CELLS. Nature. 1964 Nov 21;204:793–794. doi: 10.1038/204793a0. [DOI] [PubMed] [Google Scholar]
- Bailly S., Ferrua B., Fay M., Gougerot-Pocidalo M. A. Paraformaldehyde fixation of LPS-stimulated human monocytes: technical parameters permitting the study of membrane IL-1 activity. Eur Cytokine Netw. 1990 Mar-Apr;1(1):47–51. [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe D. M., Cotran R. S., Pober J. S. Effects of tumor necrosis factor, lipopolysaccharide, and IL-4 on the expression of vascular cell adhesion molecule-1 in vivo. Correlation with CD3+ T cell infiltration. J Immunol. 1992 Nov 1;149(9):2954–2960. [PubMed] [Google Scholar]
- Calderón E., Lockey R. F. A possible role for adhesion molecules in asthma. J Allergy Clin Immunol. 1992 Nov;90(5):852–865. doi: 10.1016/0091-6749(92)90112-f. [DOI] [PubMed] [Google Scholar]
- Charlesworth E. N., Hood A. F., Soter N. A., Kagey-Sobotka A., Norman P. S., Lichtenstein L. M. Cutaneous late-phase response to allergen. Mediator release and inflammatory cell infiltration. J Clin Invest. 1989 May;83(5):1519–1526. doi: 10.1172/JCI114047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan C. J., Kay A. B. T cells and eosinophils in the pathogenesis of asthma. Immunol Today. 1992 Dec;13(12):501–507. doi: 10.1016/0167-5699(92)90026-4. [DOI] [PubMed] [Google Scholar]
- Ebisawa M., Bochner B. S., Georas S. N., Schleimer R. P. Eosinophil transendothelial migration induced by cytokines. I. Role of endothelial and eosinophil adhesion molecules in IL-1 beta-induced transendothelial migration. J Immunol. 1992 Dec 15;149(12):4021–4028. [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. Tumour necrosis factor-like activity on paraformaldehyde-fixed monocyte monolayers. Immunology. 1987 Aug;61(4):443–448. [PMC free article] [PubMed] [Google Scholar]
- Gosset P., Tsicopoulos A., Wallaert B., Joseph M., Capron A., Tonnel A. B. Tumor necrosis factor alpha and interleukin-6 production by human mononuclear phagocytes from allergic asthmatics after IgE-dependent stimulation. Am Rev Respir Dis. 1992 Sep;146(3):768–774. doi: 10.1164/ajrccm/146.3.768. [DOI] [PubMed] [Google Scholar]
- Gundel R. H., Wegner C. D., Torcellini C. A., Clarke C. C., Haynes N., Rothlein R., Smith C. W., Letts L. G. Endothelial leukocyte adhesion molecule-1 mediates antigen-induced acute airway inflammation and late-phase airway obstruction in monkeys. J Clin Invest. 1991 Oct;88(4):1407–1411. doi: 10.1172/JCI115447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich P. C., Castell J. V., Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990 Feb 1;265(3):621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T. The biology of interleukin-6. Blood. 1989 Jul;74(1):1–10. [PubMed] [Google Scholar]
- Kriegler M., Perez C., DeFay K., Albert I., Lu S. D. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988 Apr 8;53(1):45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Beller D. I., Mizel S. B., Unanue E. R. Identification of a membrane-associated interleukin 1 in macrophages. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1204–1208. doi: 10.1073/pnas.82.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyan-Aung U., Haskard D. O., Poston R. N., Thornhill M. H., Lee T. H. Endothelial leukocyte adhesion molecule-1 and intercellular adhesion molecule-1 mediate the adhesion of eosinophils to endothelial cells in vitro and are expressed by endothelium in allergic cutaneous inflammation in vivo. J Immunol. 1991 Jan 15;146(2):521–528. [PubMed] [Google Scholar]
- Leeuwenberg J. F., von Asmuth E. J., Jeunhomme T. M., Buurman W. A. IFN-gamma regulates the expression of the adhesion molecule ELAM-1 and IL-6 production by human endothelial cells in vitro. J Immunol. 1990 Oct 1;145(7):2110–2114. [PubMed] [Google Scholar]
- Leung D. Y., Pober J. S., Cotran R. S. Expression of endothelial-leukocyte adhesion molecule-1 in elicited late phase allergic reactions. J Clin Invest. 1991 May;87(5):1805–1809. doi: 10.1172/JCI115201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., Bussolino F., Dejana E. Cytokine regulation of endothelial cell function. FASEB J. 1992 May;6(8):2591–2599. doi: 10.1096/fasebj.6.8.1592209. [DOI] [PubMed] [Google Scholar]
- Masinovsky B., Urdal D., Gallatin W. M. IL-4 acts synergistically with IL-1 beta to promote lymphocyte adhesion to microvascular endothelium by induction of vascular cell adhesion molecule-1. J Immunol. 1990 Nov 1;145(9):2886–2895. [PubMed] [Google Scholar]
- Montefort S., Feather I. H., Wilson S. J., Haskard D. O., Lee T. H., Holgate S. T., Howarth P. H. The expression of leukocyte-endothelial adhesion molecules is increased in perennial allergic rhinitis. Am J Respir Cell Mol Biol. 1992 Oct;7(4):393–398. doi: 10.1165/ajrcmb/7.4.393. [DOI] [PubMed] [Google Scholar]
- Moser R., Fehr J., Bruijnzeel P. L. IL-4 controls the selective endothelium-driven transmigration of eosinophils from allergic individuals. J Immunol. 1992 Aug 15;149(4):1432–1438. [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Osborn L. Leukocyte adhesion to endothelium in inflammation. Cell. 1990 Jul 13;62(1):3–6. doi: 10.1016/0092-8674(90)90230-c. [DOI] [PubMed] [Google Scholar]
- Parronchi P., Macchia D., Piccinni M. P., Biswas P., Simonelli C., Maggi E., Ricci M., Ansari A. A., Romagnani S. Allergen- and bacterial antigen-specific T-cell clones established from atopic donors show a different profile of cytokine production. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4538–4542. doi: 10.1073/pnas.88.10.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier R. P., Ohye R. G., Vanbuskirk A., Sedmak D. D., Kincade P., Ferguson R. M., Orosz C. G. Importance of endothelial VCAM-1 for inflammatory leukocytic infiltration in vivo. J Immunol. 1992 Oct 1;149(7):2473–2481. [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Lapierre L. A., Mendrick D. L., Fiers W., Rothlein R., Springer T. A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986 Sep 15;137(6):1893–1896. [PubMed] [Google Scholar]
- Postigo A. A., Garcia-Vicuña R., Diaz-Gonzalez F., Arroyo A. G., De Landázuri M. O., Chi-Rosso G., Lobb R. R., Laffon A., Sánchez-Madrid F. Increased binding of synovial T lymphocytes from rheumatoid arthritis to endothelial-leukocyte adhesion molecule-1 (ELAM-1) and vascular cell adhesion molecule-1 (VCAM-1). J Clin Invest. 1992 May;89(5):1445–1452. doi: 10.1172/JCI115734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleimer R. P., Sterbinsky S. A., Kaiser J., Bickel C. A., Klunk D. A., Tomioka K., Newman W., Luscinskas F. W., Gimbrone M. A., Jr, McIntyre B. W. IL-4 induces adherence of human eosinophils and basophils but not neutrophils to endothelium. Association with expression of VCAM-1. J Immunol. 1992 Feb 15;148(4):1086–1092. [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Streck H., Günther C., Beuscher H. U., Röllinghoff M. Studies on the release of cell-associated interleukin 1 by paraformaldehyde-treated murine macrophages. Eur J Immunol. 1988 Oct;18(10):1609–1613. doi: 10.1002/eji.1830181021. [DOI] [PubMed] [Google Scholar]
- Thornhill M. H., Kyan-Aung U., Haskard D. O. IL-4 increases human endothelial cell adhesiveness for T cells but not for neutrophils. J Immunol. 1990 Apr 15;144(8):3060–3065. [PubMed] [Google Scholar]
- Thornhill M. H., Wellicome S. M., Mahiouz D. L., Lanchbury J. S., Kyan-Aung U., Haskard D. O. Tumor necrosis factor combines with IL-4 or IFN-gamma to selectively enhance endothelial cell adhesiveness for T cells. The contribution of vascular cell adhesion molecule-1-dependent and -independent binding mechanisms. J Immunol. 1991 Jan 15;146(2):592–598. [PubMed] [Google Scholar]
- Van Snick J., Cayphas S., Vink A., Uyttenhove C., Coulie P. G., Rubira M. R., Simpson R. J. Purification and NH2-terminal amino acid sequence of a T-cell-derived lymphokine with growth factor activity for B-cell hybridomas. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9679–9683. doi: 10.1073/pnas.83.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh G. M., Mermod J. J., Hartnell A., Kay A. B., Wardlaw A. J. Human eosinophil, but not neutrophil, adherence to IL-1-stimulated human umbilical vascular endothelial cells is alpha 4 beta 1 (very late antigen-4) dependent. J Immunol. 1991 May 15;146(10):3419–3423. [PubMed] [Google Scholar]
- Weg V. B., Williams T. J., Lobb R. R., Nourshargh S. A monoclonal antibody recognizing very late activation antigen-4 inhibits eosinophil accumulation in vivo. J Exp Med. 1993 Feb 1;177(2):561–566. doi: 10.1084/jem.177.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner C. D., Gundel R. H., Reilly P., Haynes N., Letts L. G., Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) in the pathogenesis of asthma. Science. 1990 Jan 26;247(4941):456–459. doi: 10.1126/science.1967851. [DOI] [PubMed] [Google Scholar]


