Figure 1.
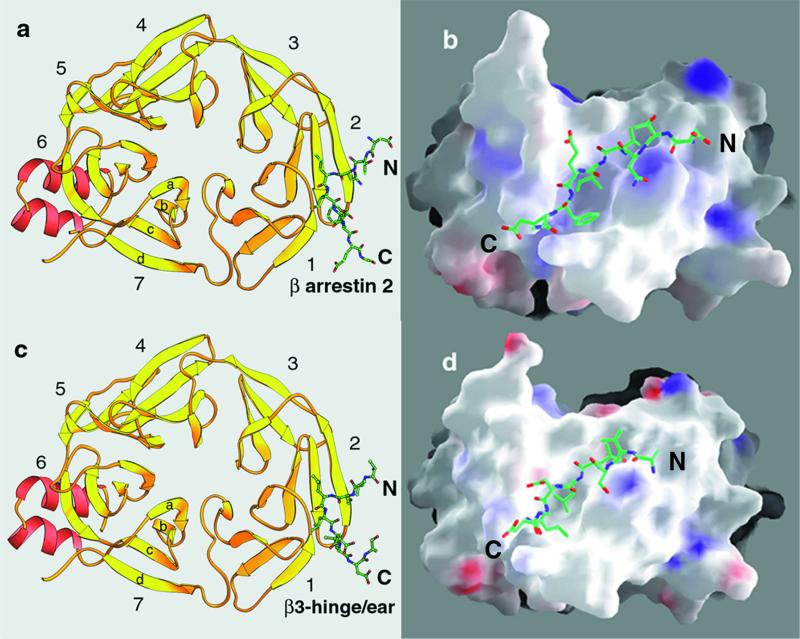
Structures of clathrin-terminal domain complexed with clathrin-box peptides from β-arrestin 2 (a and b) and β3-hinge of AP-3 (c and d). (a and c) Ribbon diagrams of the complexes. The representation is a “top” view of the td40 fragment (residues 3–359) looking from the membrane toward the surrounding clathrin coat. The propeller blades are numbered 1–7; each blade contains four antiparallel β-strands, labeled a–d. Ordered portions of the bound peptides from β-arrestin 2 and β3-hinge (of sequence DTNLIEFE and VSLLDLD) are in green. The figure was made with ribbons (31). (b and d) Surface representation of the complexes. The images of b and d have been rotated approximately 90° toward the viewer. The map shows positive (blue), negative (red), and hydrophobic/neutral patches (white) projected onto the surface representation. The figure was made with grasp (32).