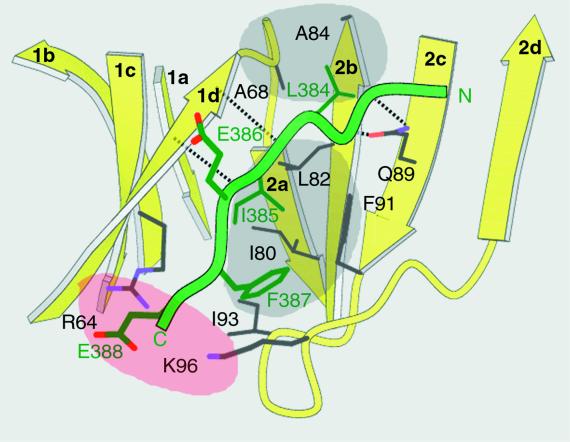
Figure 3.
Close-up view of the peptide-in-groove interactions between the clathrin box in the peptide of β-arrestin 2 and the clathrin-terminal domain. The residues that contribute to the interaction between the β-arrestin 2 peptide and the clathrin groove between blades 1 and 2 are labeled by single-letter code and by the positions in their respective sequences. The dashed lines show hydrogen bonds between the backbones of blade 1d and bound peptide and between peptide backbone and the side chain of clathrin Q89. The locations for the two hydrophobic (gray) and polar (rose) pockets in the clathrin groove are approximate.