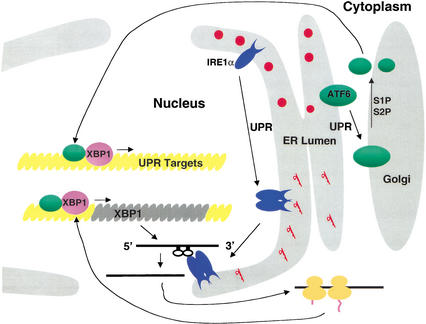
Figure 7.
ATF6- and IRE1α-dependent UPR signaling pathways merge through regulation of the quantity and quality, respectively, of XBP1 protein. The model depicts the activation of two proximal sensors of the UPR, ATF6 and IRE1α, upon ER stress. Upon accumulation of unfolded proteins in the ER lumen, ATF6 leaves the ER to enter the Golgi apparatus, where it is cleaved by S1P and then S2P to release a 50-kD fragment that enters the nucleus through the nuclear pore. p50-ATF6 then interacts with ERSE motifs to activate transcription. Simultaneously and independently, the UPR induces dimerization, autophosphorylation, and activation of the RNase activity of IRE1α that is localized at the inner leaflet of the nuclear envelope. Activated IRE1α then initiates splicing of XBP1 mRNA to generate a potent transcriptional activator, XBP1-s, that also enters the nuclear pore to activate transcription from ERSE motifs. The status of XBP1-s and p50-ATF6 when bound to the ERSE is not known, but for simplicity they are depicted as heterodimers.