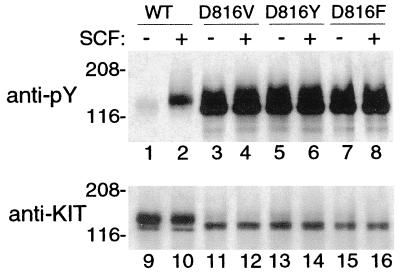
Figure 1.
KITWT and KIT mutant phosphorylation in COS-7 cells. Blotting of immunoprecipitated KIT with an antiphosphotyrosine antibody (anti-PY, lanes 1–8) confirms in our system the previously reported results of other researchers (23, 24, 32) showing minimal spontaneous tyrosine phosphorylation of KIT in COS-7 cells expressing KITWT (lane 1) and high levels of spontaneous tyrosine phosphorylation of mutant KITs with substitution in position 816 (lanes 3, 5, and 7). These results serve as controls for Fig. 2. Increased tyrosine phosphorylation of KITWT is seen after exposure to exogenous SCF (+, lane 2), but tyrosine phosphorylation of codon 816 mutants is already maximal, so there is no increase in response to SCF (compare lanes 4, 6, and 8 with lanes 3, 5, and 7). After stripping, reprobing of the blot with anti-KIT antibody demonstrates the relative amounts of protein in each lane (lanes 9–16). Note that much greater amounts of KITWT are needed to demonstrate spontaneous tyrosine phosphorylation than are needed with codon 816 mutants (compare lane 9 with lanes 11, 13, and 15) and that maximal KIT tyrosine phosphorylation is higher in the mutant KITs than in KITWT (compare lane pairs 2 and 10 with 4 and 12, 6 and 14, and 8 and 16).