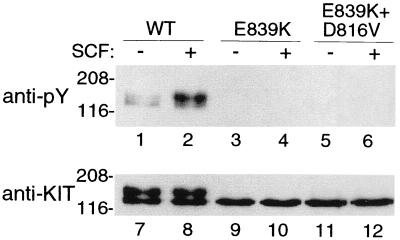
Figure 2.
Codon 839 mutation inactivates KIT. Blotting of immunoprecipitated KIT with an antiphosphotyrosine antibody (anti-Py, lanes 1–6) shows a low level of spontaneous KIT tyrosine phosphorylation in COS-7 cells expressing high levels of KITWT (lane 1) but no spontaneous phosphorylation of KITE839K (lane 3). KITWT is phosphorylated in response to exogenous SCF, but KITE839K is not (lanes 2 and 4, respectively). A double mutant, KITD816V/E839K, which incorporates both the activating D816V mutation and E839K loss-of-function mutation, is not phosphorylated spontaneously or in response to exogenous SCF, indicating that the E839K has an intramolecular dominant–inactivating effect (lanes 5 and 6). Blotting with anti-KIT antibody after stripping the antiphosphotyrosine antibody (anti-KIT, lanes 7–12) shows KIT protein in all transfectants. Note also that KITWT is present as both a 125-kDa form and as a fully glycosylated, mature, 145-kDa form (lanes 7 and 8) but that KITE839K is present predominately as a lower molecular weight form in the steady state (lanes 9, 10, 11, and 12).