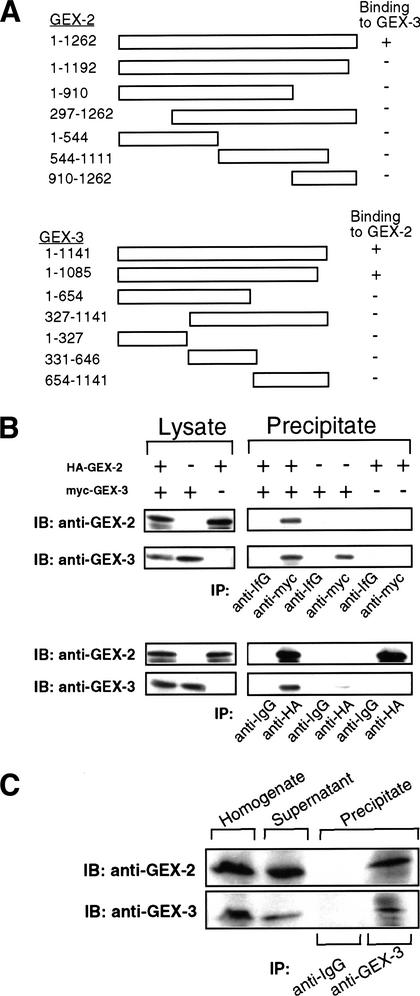
Figure 6.
Direct interaction between GEX-2 and GEX-3. (A) Two-hybrid scheme including deletion constructs. The GEX-2 deletion series fused to the GAL4 activator domain and full-length GEX-3 fused to the GAL4 DNA-binding domain were tested in the two-hybrid assay. Only the combination of full-length GEX-2 and GEX-3 shows a direct interaction. Reciprocally, the GEX-3 deletion series fused to the GAL4 DNA-binding domain and full-length GEX-2 fused to the GAL4 activator domain were tested for interaction in the two-hybrid assay. Full-length and GEX-3 slightly deleted at the C terminus could bind to full-length GEX-2 in the two-hybrid assay. (B) Immunoprecipitation from yeast. Yeast lysates expressing HA–GEX-2 and myc–GEX-3 were prepared as described in Materials and Methods. Left lysate panels shows the expression of HA–GEX-2 and myc–GEX-3 recombinant proteins in yeast. These lysates were immunoprecipitated with anti-myc antibody (upper panels) and anti-HA antibody (lower panels). Coprecipitation was observed in the precipitate of the lysate prepared from yeast expressing both HA–GEX-2 and myc–GEX-3 as detected by anti-GEX-2 and anti-GEX-3 antibodies. (IB) Immunoblot; (IP) immunoprecipitate. (C) Immunoprecipitation from C. elegans. C. elegans lysates were immunoprecipitated with antibodies to GEX-3 and probed with anti-GEX-2 and anti-GEX-3 rabbit polyclonal antibodies. The GEX-3 immunoprecipitation includes a protein that can be detected by the GEX-2 antibody.