Abstract
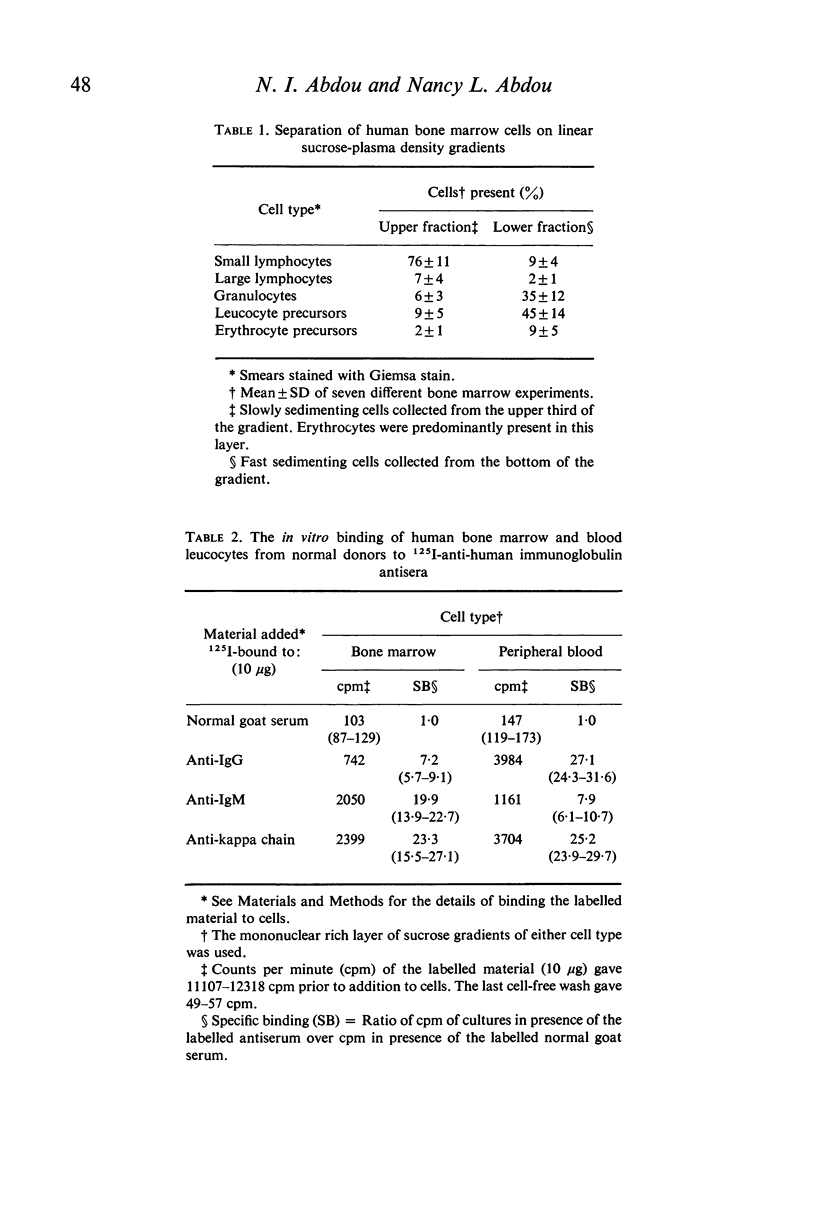
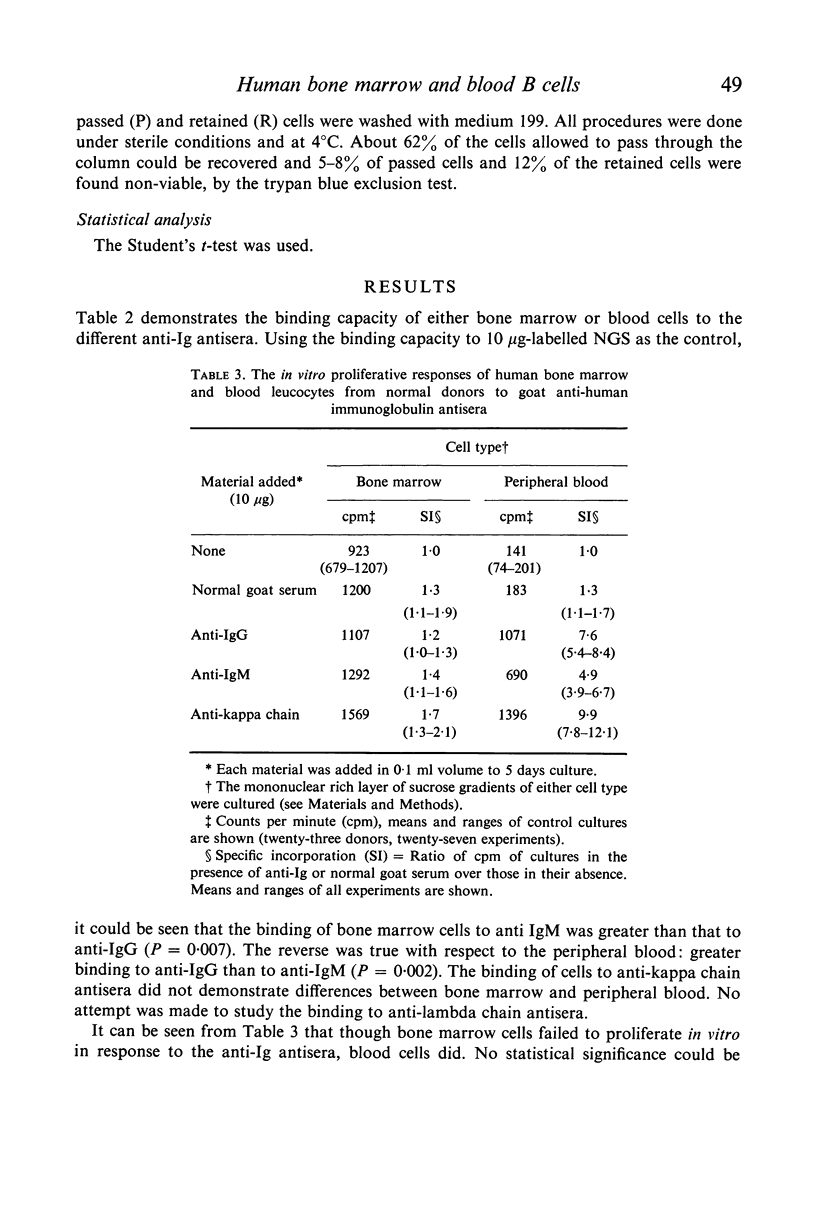
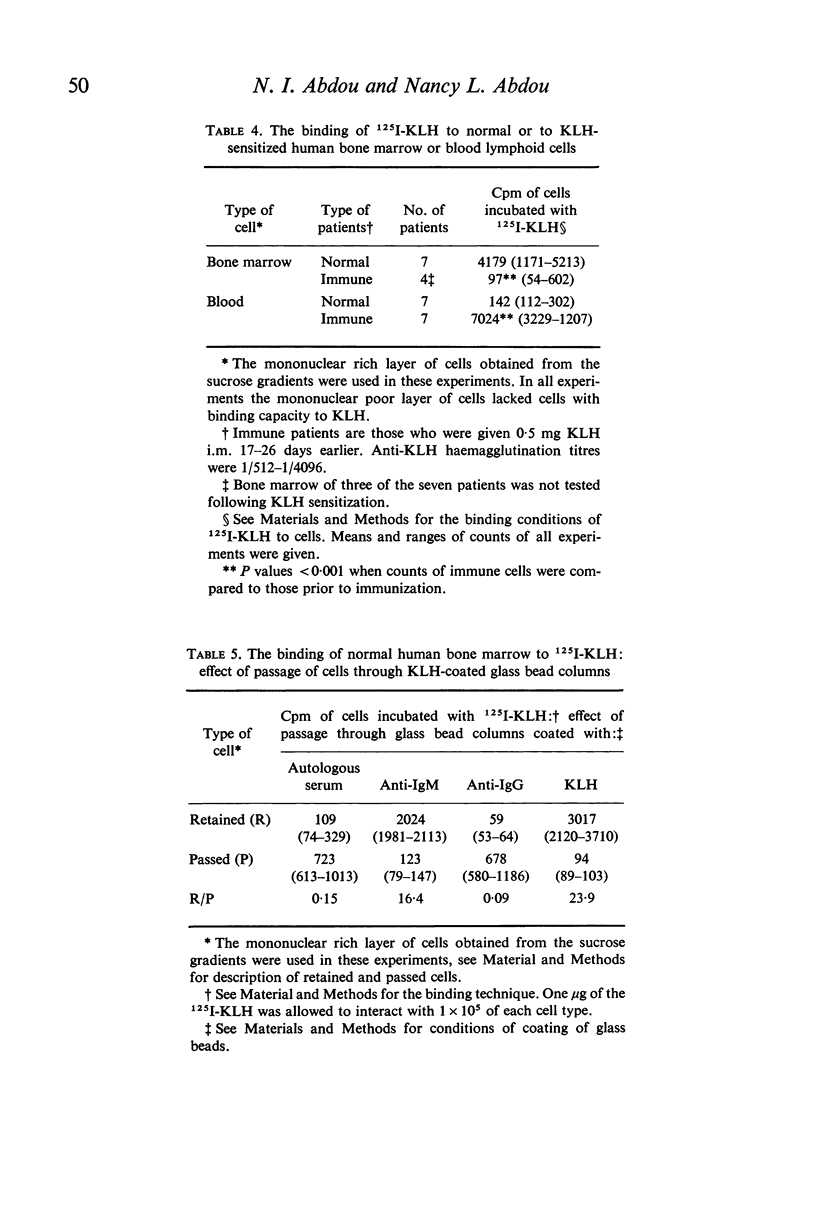
A comparative study of B cells present in human bone marrow and blood was performed. In both compartments the cells carrying the Ig receptors were found to be small mononuclear cells. Predominance of IgM receptors was found on bone marrow cells whereas Ig receptors present on peripheral blood cells were predominantly of the IgG class. Bone marrow lymphoid cells of non-sensitized donors were capable of binding a primary antigen, keyhole limpet haemocyanin (KLH) and could be retained on glass bead columns coated with either KLH or with goat anti-human IgM antiserum but not with anti-IgG. Whereas bone marrow cells of donors immunized with KLH 16–27 days earlier lacked KLH reactive cells, the latter cells could be demonstrated in the blood. It is concluded that human bone marrow B cells carrying IgM receptors are essential for the early antigen recognition step following which recruitment of these cells into the circulation takes place.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., Abdou N. L. Bone marrow: the bursa equivalent in man? Science. 1972 Jan 28;175(4020):446–448. doi: 10.1126/science.175.4020.446. [DOI] [PubMed] [Google Scholar]
- Abdou N. I. Immunoglobulin (Ig) receptors on human peripheral leukocytes. II. Class restriction Ig receptors. J Immunol. 1971 Dec;107(6):1637–1642. [PubMed] [Google Scholar]
- Abdou N. I., Richter M. Cells involved in the immune response, IX. Depletion from the normal rabbit bone marrow of antigen-reactive cells directed toward human peripheral leukocytes. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1136–1143. doi: 10.1073/pnas.63.4.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdou N. I., Richter M. Cells involved in the immune response. VI. The immune response to red blood cells in irradiated rabbits after administration of normal, primed, or immune allogeneic rabbit bone marrow cells. J Exp Med. 1969 Apr 1;129(4):757–774. doi: 10.1084/jem.129.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdou N. I., Richter M. Cells involved in the immune response. X. The transfer of antibody-forming capacity to irradiated rabbits by antigen-reactive cells isolated from normal allogeneic rabbit bone marrow after passage through antigen-sensitized glass bead columns. J Exp Med. 1969 Jul 1;130(1):141–163. doi: 10.1084/jem.130.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdou N. I., Richter M. The role of bone marrow in the immune response. Adv Immunol. 1970;12:201–270. doi: 10.1016/s0065-2776(08)60170-4. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Basten A., Miller J. F., Warner N. L., Pye J. Specific inactivation of thymus-derived (T) and non-thymus-derived (B) lymphocytes by 125I-labelled antigen. Nat New Biol. 1971 May 26;231(21):104–106. doi: 10.1038/newbio231104a0. [DOI] [PubMed] [Google Scholar]
- Basten A., Warner N. L., Mandel T. A receptor for antibody on B lymphocytes. II. Immunochemical and electron microscopy characteristics. J Exp Med. 1972 Mar 1;135(3):627–642. doi: 10.1084/jem.135.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis J. E., Hersh E. M., Butler W. T., Rossen R. D. Antigen dose in the human immune response. Dose-relationships in the human immune response to Keyhole limpet hemocyanin. J Lab Clin Med. 1971 Jul;78(1):61–69. [PubMed] [Google Scholar]
- Daguillard F., Richter M. Cells involved in the immune response. XII. The differing responses of normal rabbit lymphoid cells to phytohemagglutinin, goat anti-rabbit immunoglobulin antiserum and allogeneic and xenogeneic lymphocytes. J Exp Med. 1969 Nov 1;130(5):1187–1208. doi: 10.1084/jem.130.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie J. M., Paul W. E. Receptors on immunocompetent cells. V. Cellular correlates of the "maturation" of the immune response. J Exp Med. 1972 Mar 1;135(3):660–674. doi: 10.1084/jem.135.3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer J. M., Warner N. L. Antigen binding cells in embryonic chicken bursa and thymus. Nat New Biol. 1971 Feb 17;229(7):210–211. doi: 10.1038/newbio229210a0. [DOI] [PubMed] [Google Scholar]
- Fröland S., Natvig J. B., Berdal P. Surface-bound immunoglobulin as a marker of B lymphocytes in man. Nat New Biol. 1971 Dec 22;234(51):251–252. doi: 10.1038/newbio234251a0. [DOI] [PubMed] [Google Scholar]
- Grey H. M., Rabellino E., Pirofsky B. Immunoglobulins on the surface of lymphocytes. IV. Distribution in hypogammaglobulinemia, cellular immune deficiency, and chronic lymphatic leukemia. J Clin Invest. 1971 Nov;50(11):2368–2375. doi: 10.1172/JCI106735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton A. R., 3rd, Asofsky R., Hylton M. B., Cooper M. D. Suppression of immunoglobulin class synthesis in mice. I. Effects of treatment with antibody to -chain. J Exp Med. 1972 Feb 1;135(2):277–297. doi: 10.1084/jem.135.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- McCulloch E. A., Till J. E. Regulatory mechanisms acting on hemopoietic stem cells. Some clinical implications. Am J Pathol. 1971 Dec;65(3):601–619. [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Warner N. L., Lewis H., Sprent J. Quantitative features of a sandwich radioimmunolabeling technique for lymphocyte surface receptors. J Exp Med. 1972 Feb 1;135(2):405–428. doi: 10.1084/jem.135.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott D. M., De Sousa M. Thymus-dependent and thymus-independent populations: origin, migratory patterns and lifespan. Clin Exp Immunol. 1971 May;8(5):663–684. [PMC free article] [PubMed] [Google Scholar]
- Perkins W. D., Karnovsky M. J., Unanue E. R. An ultrastructural study of lymphocytes with surface-bound immunoglobulin. J Exp Med. 1972 Feb 1;135(2):267–276. doi: 10.1084/jem.135.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabellino E., Grey H. M. Immunoglobulins on the surface of lymphocytes. 3. Bursal origin of surface immunoglobulins on chicken lymphocytes. J Immunol. 1971 May;106(5):1418–1420. [PubMed] [Google Scholar]
- Raff M. C. T and B lymphocytes in mice studied by using antisera against surface antigenic markers. Am J Pathol. 1971 Nov;65(2):467–478. [PMC free article] [PubMed] [Google Scholar]
- Singhal S. K., Wigzell H. Cognition and recognition of antigen by cell associated receptors. Prog Allergy. 1971;15:188–222. [PubMed] [Google Scholar]
- Sprent J., Miller J. F., Mitchell G. F. Antigen-induced selective recruitment of circulating lymphocytes. Cell Immunol. 1971 Apr;2(2):171–181. doi: 10.1016/0008-8749(71)90036-0. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Baur S., Uhr J. W. Cell surface immunoglobulin. II. Isolation and characterization of immunoglobulin from mouse splenic lymphocytes. J Exp Med. 1971 Jul 1;134(1):242–264. doi: 10.1084/jem.134.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. D., Nossal G. J. Identification of human T and B lymphocytes in normal peripheral blood and in chronic lymphocytic leukaemia. Lancet. 1971 Oct 9;2(7728):788–791. doi: 10.1016/s0140-6736(71)92741-3. [DOI] [PubMed] [Google Scholar]


