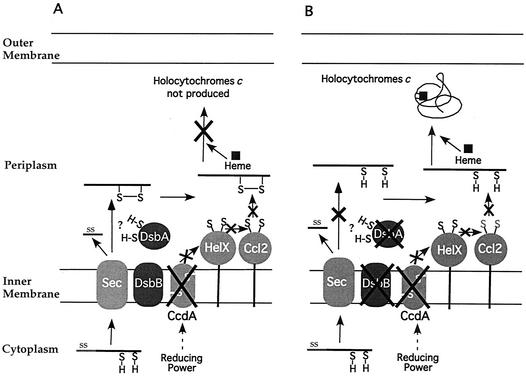
FIG. 6.
A model to illustrate how the inactivation of DsbA or DsbB could restore the biogenesis of c-type Cyts in a CcdA-null mutant of R. capsulatus. Apo-Cyts are translocated across the membrane by the Sec-dependent general secretory pathway (Sec). Upon entry into the periplasm, their signal sequences (SS) are cleaved by the signal peptidase (not shown). A disulfide bond (S—S) between the heme-binding cysteines (S=H) is thought to be formed by the DsbA-DsbB system as the apo-Cyts emerge into the periplasm. This disulfide bond must be subsequently reduced for the apo-Cyts to be competent for heme ligation. The reducing power required for this process is thought to be shuttled across the membrane via CcdA and then relayed through HelX and Ccl2 to the apo-Cyts. In the absence of CcdA and the presence of DsbA-DsbB (panel A), the cysteines in the heme-binding site of the apo-Cyts form a disulfide bond and thus remain inaccessible for heme ligation. When either DsbA or DsbB is absent in addition to CcdA (panel B), the cysteines in the heme-binding site remain reduced and hence available for heme ligation. For the sake of simplicity, other proteins involved in the biogenesis of c-type Cyts in R. capsulatus are not shown. A more complete illustration of this process is presented elsewhere (14).