Abstract
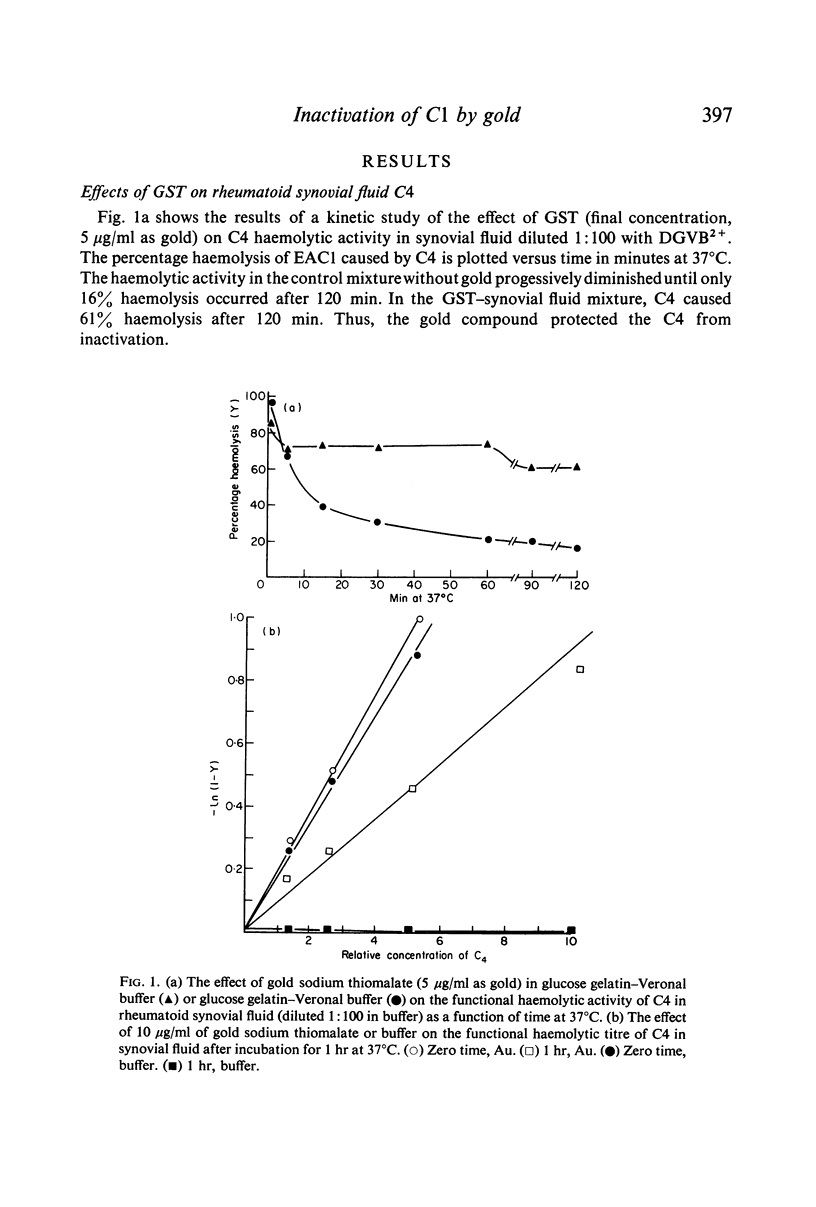
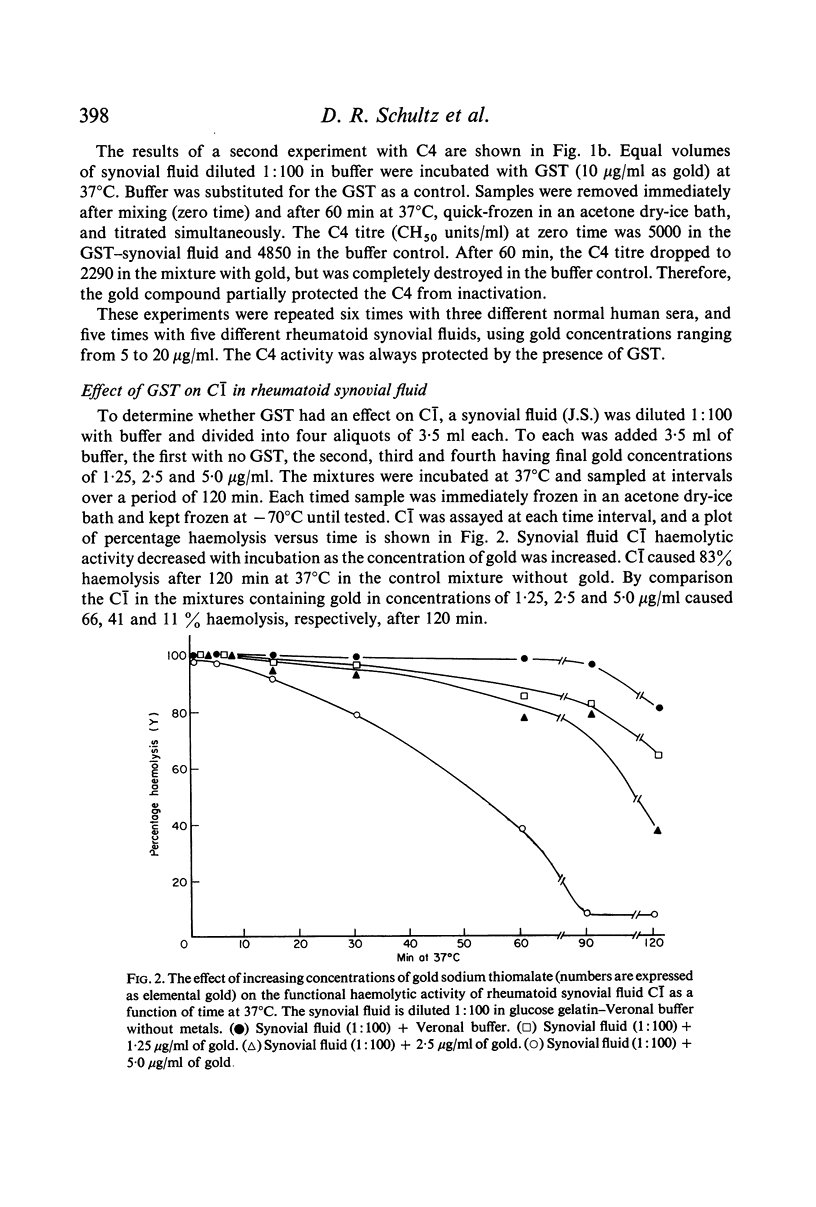
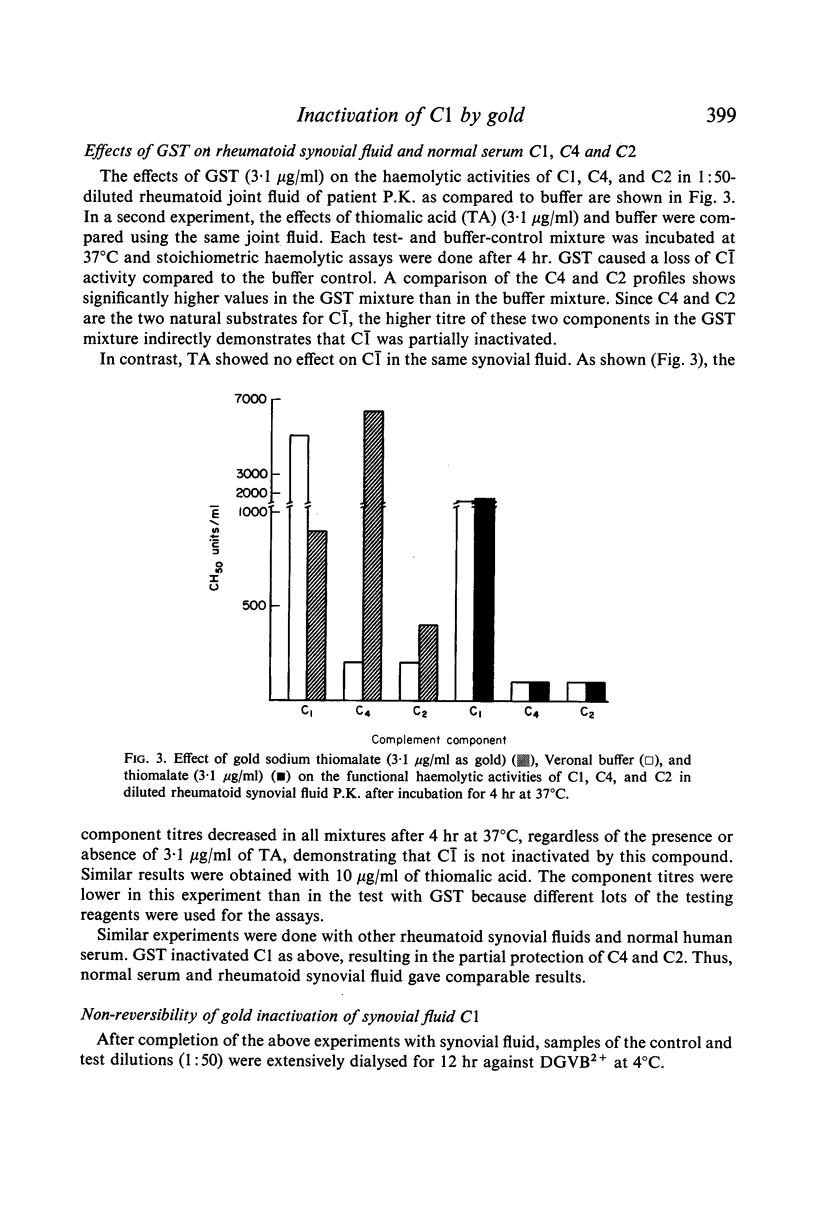
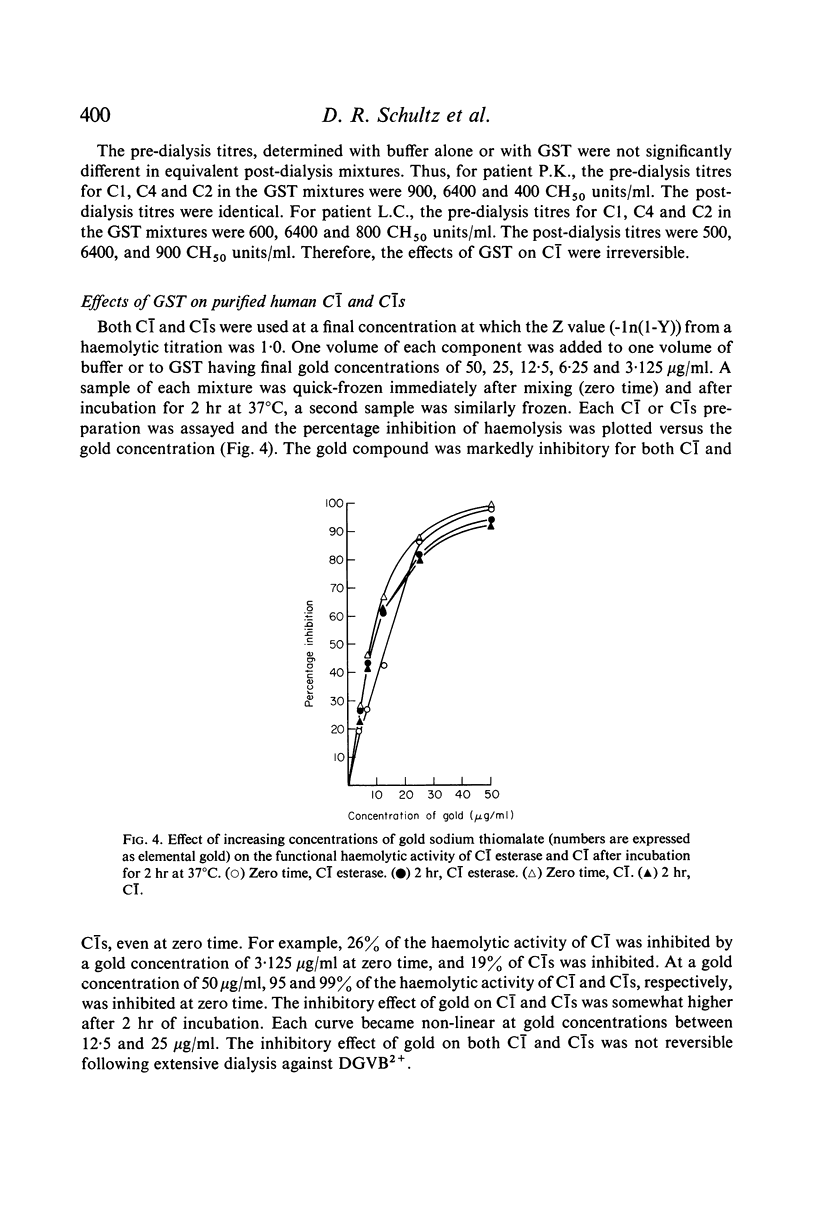
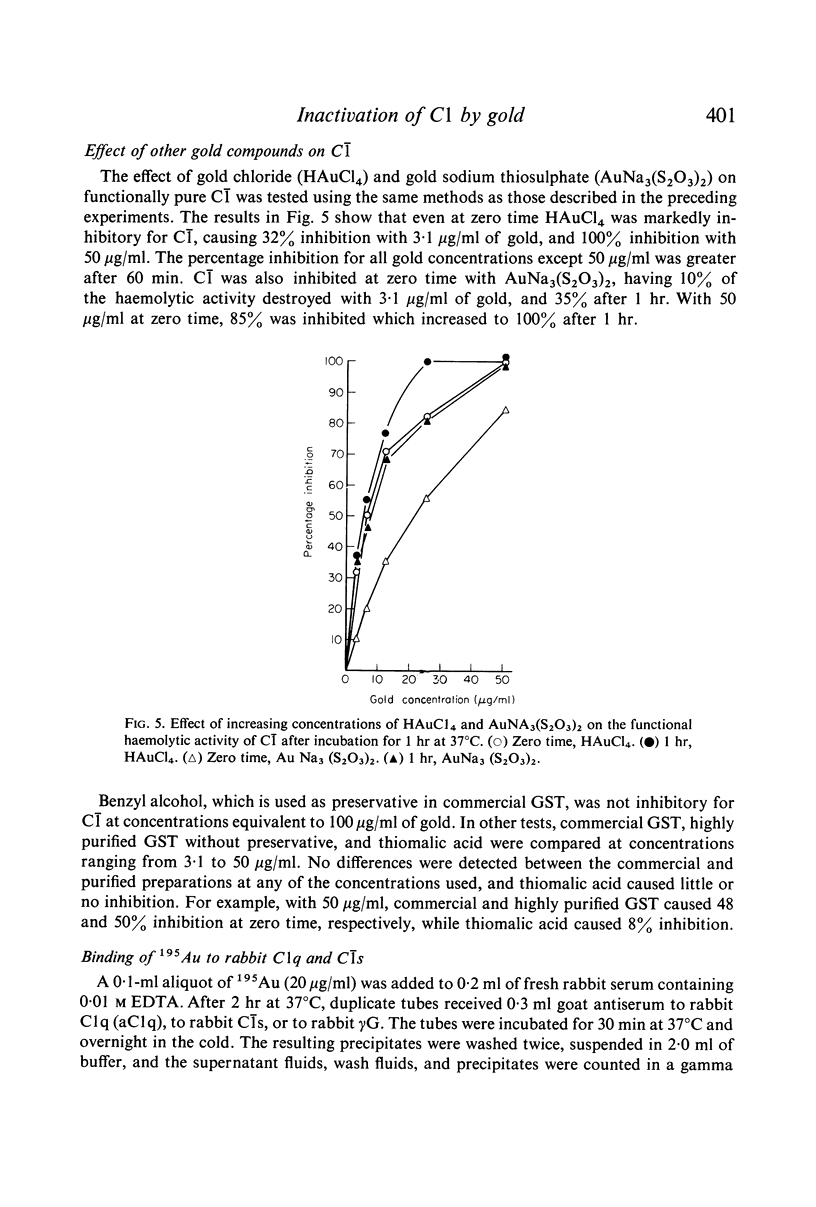
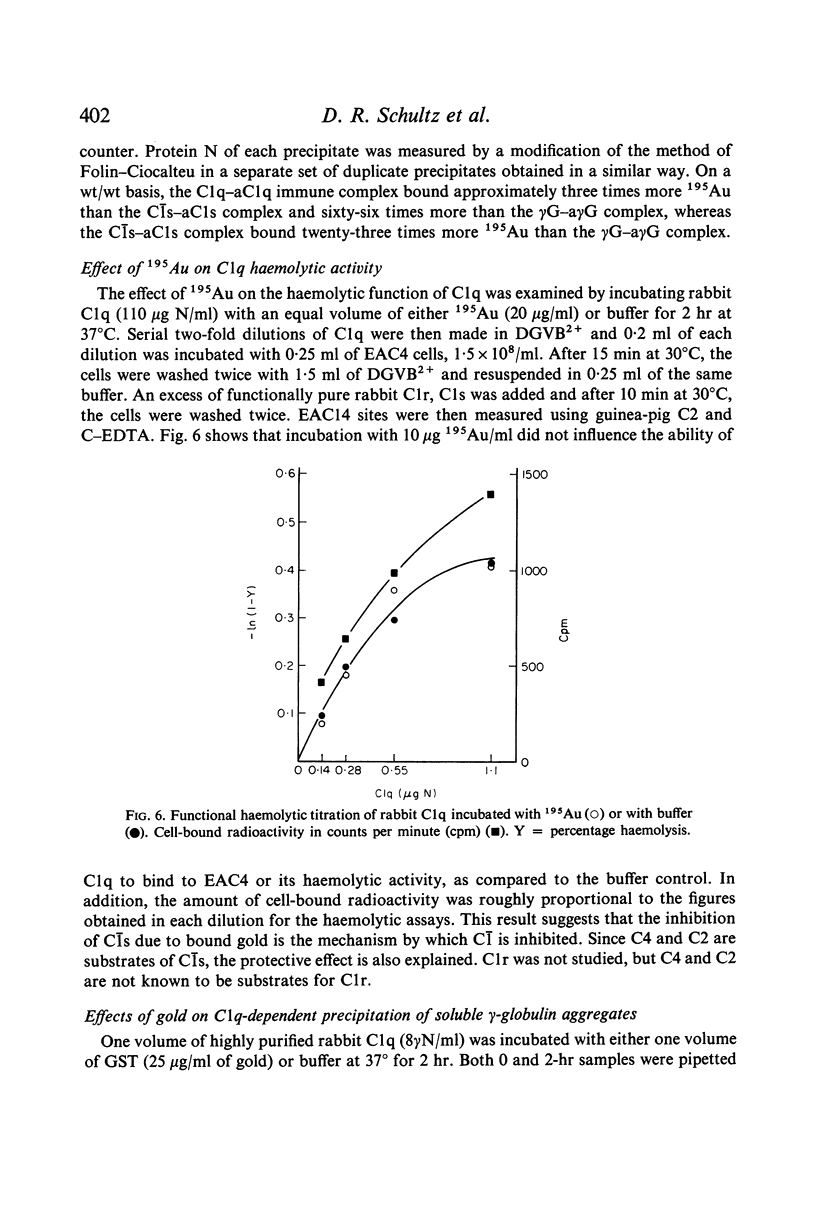
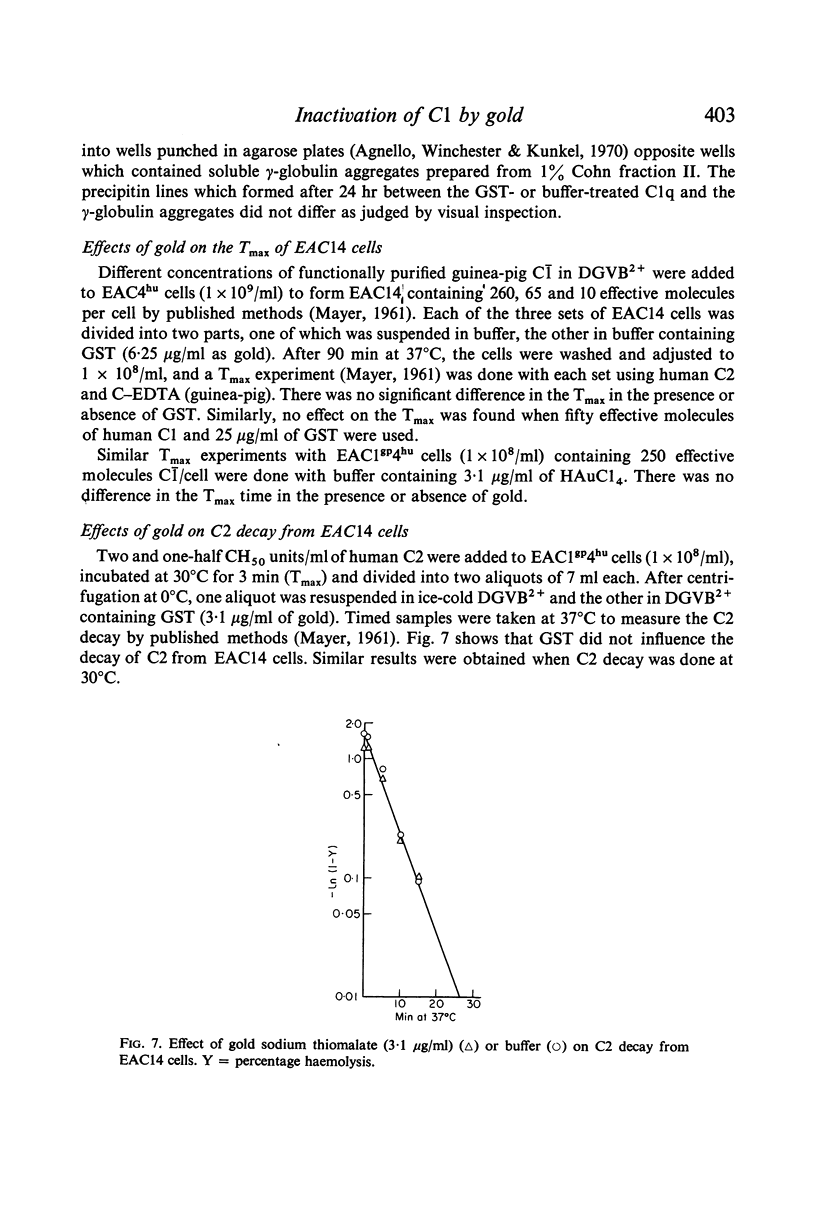
The effects of gold compounds on the functional activities of rheumatoid synovial fluid and normal serum Cl, C4, and C2 were investigated in vitro. Commercial and purified gold sodium thiomalate in concentrations, as low as 1·25 μg/ml (expressed as elemental gold) inactivated native C[unk] and highly purified C[unk]s, whereas equivalent or higher concentrations of thiomalate had no effect. C[unk] inactivation was caused also by other gold compounds such as gold chloride and gold sodium thiosulphate. The C[unk] inactivation was not reversible following extensive dialysis. The partial protection of C4 and C2, the two natural substrates for C[unk], indirectly verified the C[unk] inactivation. This is the first study to show that gold compounds inactivate C[unk], one of the reactants in the pathogenesis and/or perpetuation of rheumatoid arthritis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnello V., Winchester R. J., Kunkel H. G. Precipitin reactions of the C1q component of complement with aggregated gamma-globulin and immune complexes in gel diffusion. Immunology. 1970 Dec;19(6):909–919. [PMC free article] [PubMed] [Google Scholar]
- DONALDSON V. H., EVANS R. R. A BIOCHEMICAL ABNORMALITY IN HEREDIATRY ANGIONEUROTIC EDEMA: ABSENCE OF SERUM INHIBITOR OF C' 1-ESTERASE. Am J Med. 1963 Jul;35:37–44. doi: 10.1016/0002-9343(63)90162-1. [DOI] [PubMed] [Google Scholar]
- DONALDSON V. H., ROSEN F. S. ACTION OF COMPLEMENT IN HEREDITARY ANGIONEUROTIC EDEMA: THE ROLE OF C'1-ESTERASE. J Clin Invest. 1964 Nov;43:2204–2213. doi: 10.1172/JCI105094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan R. E., Day N. K., Luckasen J. R., Good R. A. Complement activation in pemphigus vulgaris blister fluid. Clin Exp Immunol. 1973 Sep;15(1):53–63. [PMC free article] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Chemistry and reaction mechanisms of complement. Adv Immunol. 1968;8:1–80. doi: 10.1016/s0065-2776(08)60464-2. [DOI] [PubMed] [Google Scholar]
- Persellin R. H., Ziff M. The effect of gold salt on lysosomal enzymes of the peritoneal macrophage. Arthritis Rheum. 1966 Feb;9(1):57–65. doi: 10.1002/art.1780090107. [DOI] [PubMed] [Google Scholar]
- Ruddy S., Austen K. F. Activation of the complement system in rheumatoid synovitis. Fed Proc. 1973 Feb;32(2):134–137. [PubMed] [Google Scholar]
- Stroud R. M., Austen K. F., Mayer M. M. Catalysis of C'2 fixation by C'la. Reaction kinetics, competitive inhibition by TAMe, and transferase hypothesis of the enzymatic action of C'la on C'2, one of its natural substrates. Immunochemistry. 1965 Sep;2(3):219–234. [PubMed] [Google Scholar]
- Tamura N., Nelson R. A., Jr The purification and reactivity of the first component of complement from guinea pig, human and canine sera. J Immunol. 1968 Dec;101(6):1333–1345. [PubMed] [Google Scholar]
- Volanakis J. E., Stroud R. M. Rabbit C1q: purification, functional and structural studies. J Immunol Methods. 1972 Nov;2(1):25–34. doi: 10.1016/0022-1759(72)90014-2. [DOI] [PubMed] [Google Scholar]
- Vroon D. H., Schultz D. R., Zarco R. M. The separation of nine components and two inactivators of components of complement in humansserum. Immunochemistry. 1970 Jan;7(1):43–61. doi: 10.1016/0019-2791(70)90029-7. [DOI] [PubMed] [Google Scholar]


