Abstract
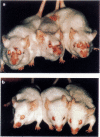
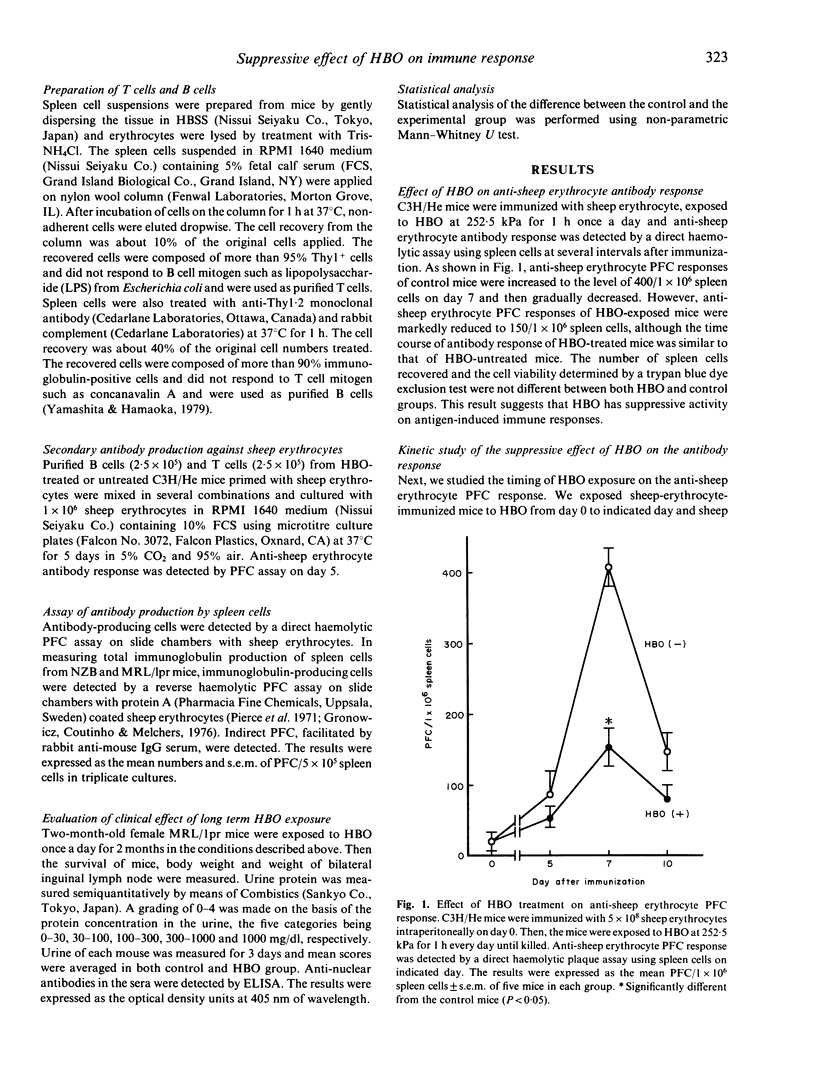
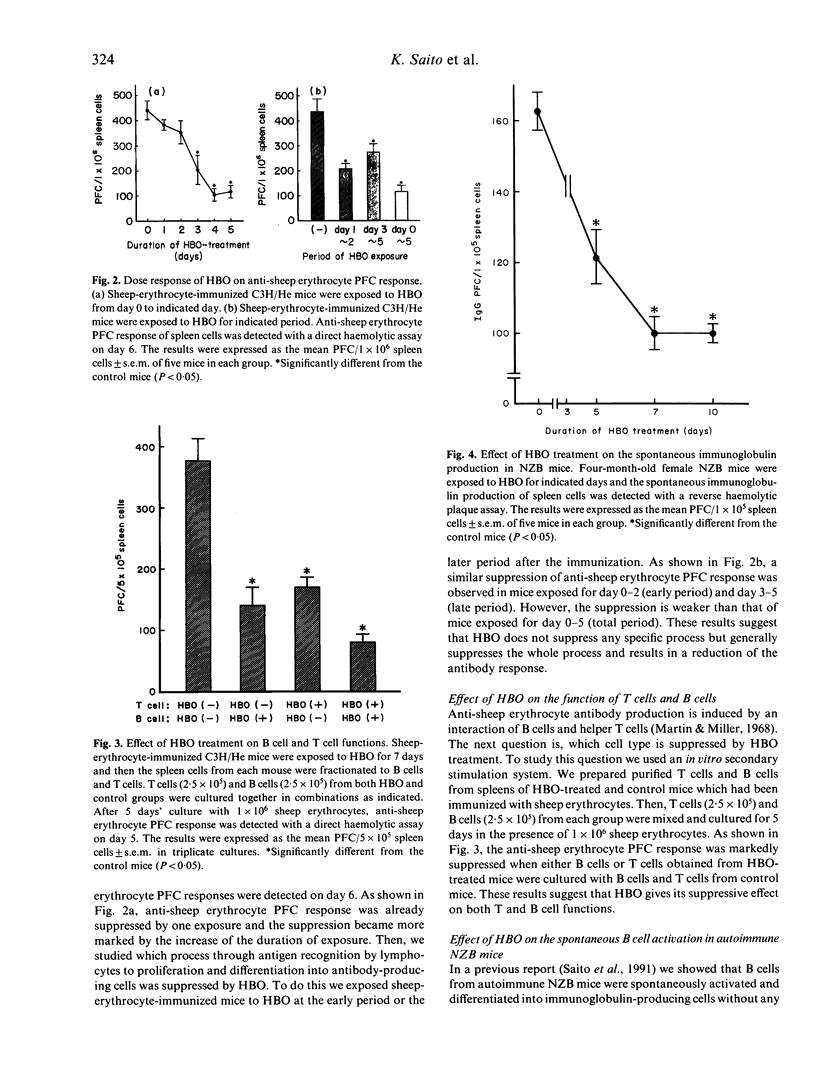
We studied the effect of hyperbaric oxygenation (HBO) on immune responses in normal and autoimmune mice. Mice were exposed to HBO in an animal chamber at a pressure of 252.5 kPa for 1 h and once a day for 5 days. The immunization of C3H/He mice with sheep erythrocytes induced marked anti-sheep erythrocyte antibody response on day 7. However, this response was markedly suppressed in HBO-treated mice. The suppression is dependent on the duration of HBO and it works on the early and the late stage of antibody responses. HBO suppresses the development of both sheep erythrocyte-specific B cells and helper T cells after the immunization. Then, we tried to expose autoimmune mice to HBO. Spontaneous immunoglobulin production of NZB and MRL/lpr spleen cells was also significantly suppressed by HBO. Furthermore, long term HBO exposure results in the suppression of the development of autoimmune symptoms such as proteinuria, facial erythema and lymphadenopathy in MRL/lpr mice. All these results suggest that HBO is applicable for the treatment of autoimmune diseases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEAN J. W., SMITH C. W. Hypophyseal and adrenocoritcal factors in pulmonary damage induced by oxygen at atmospheric pressure. Am J Physiol. 1953 Jan;172(1):169–174. doi: 10.1152/ajplegacy.1952.172.1.169. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976 Aug;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- Hoffeld J. T. Agents which block membrane lipid peroxidation enhance mouse spleen cell immune activities in vitro: relationship to the enhancing activity of 2-mercaptoethanol. Eur J Immunol. 1981 May;11(5):371–376. doi: 10.1002/eji.1830110505. [DOI] [PubMed] [Google Scholar]
- Izui S., McConahey P. J., Dixon F. J. Increased spontaneous polyclonal activation of B lymphocytes in mice with spontaneous autoimmune disease. J Immunol. 1978 Dec;121(6):2213–2219. [PubMed] [Google Scholar]
- Martin W. J., Miller J. F. Cell to cell interaction in the immune response. IV. Site of action of antilymphocyte globulin. J Exp Med. 1968 Oct 1;128(4):855–874. doi: 10.1084/jem.128.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey S. A., Hyams J. S., Fisher A. B., Root R. K. Effects of oxygen exposure on it vitro function of pulmonary alveolar macrophages. J Clin Invest. 1975 Aug;56(2):503–511. doi: 10.1172/JCI108117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce C. W., Johnson B. M., Gershon H. E., Asofsky R. Immune responses in vitro. 3. Development of primary gamma-M, gamma-G, and gamma-A plaque-forming cell responses in mouse spleen cell cultures stimulated with heterologous erythrocytes. J Exp Med. 1971 Aug 1;134(2):395–416. doi: 10.1084/jem.134.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prud'Homme G. J., Park C. L., Fieser T. M., Kofler R., Dixon F. J., Theofilopoulos A. N. Identification of a B cell differentiation factor(s) spontaneously produced by proliferating T cells in murine lupus strains of the lpr/lpr genotype. J Exp Med. 1983 Feb 1;157(2):730–742. doi: 10.1084/jem.157.2.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prud'homme G. J., Balderas R. S., Dixon F. J., Theofilopoulos A. N. B cell dependence on and response to accessory signals in murine lupus strains. J Exp Med. 1983 Jun 1;157(6):1815–1827. doi: 10.1084/jem.157.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedor J. R., Carey S. W., Emancipator S. N. Immune complexes bind to cultured rat glomerular mesangial cells to stimulate superoxide release. Evidence for an Fc receptor. J Immunol. 1987 Jun 1;138(11):3751–3757. [PubMed] [Google Scholar]
- Steinberg A. D., Roths J. B., Murphy E. D., Steinberg R. T., Raveche E. S. Effects of thymectomy or androgen administration upon the autoimmune disease of MRL/Mp-lpr/lpr mice. J Immunol. 1980 Aug;125(2):871–873. [PubMed] [Google Scholar]
- Warren J. S., Kunkel S. L., Johnson K. J., Ward P. A. In vitro activation of rat neutrophils and alveolar macrophages with IgA and IgG immune complexes. Implications for immune complex-induced lung injury. Am J Pathol. 1987 Dec;129(3):578–588. [PMC free article] [PubMed] [Google Scholar]
- Warren J., Sacksteder M. R., Thuning C. A. Oxygen immunosuppression: modification of experimental allergic encephalomyelitis in rodents. J Immunol. 1978 Jul;121(1):315–320. [PubMed] [Google Scholar]
- Warren J., Sacksteder M. R., Thuning C. A. Therapeutic effect of prolonged hyperbaric oxygen in adjuvant arthritis of the rat. Arthritis Rheum. 1979 Apr;22(4):334–339. doi: 10.1002/art.1780220404. [DOI] [PubMed] [Google Scholar]
- Yamashita U., Hamaoka T. The requirement of Ia-positive accessory cells for the induction of hapten-reactive cytotoxic T lymphocytes in vitro. J Immunol. 1979 Dec;123(6):2637–2643. [PubMed] [Google Scholar]