Abstract
Anti-neutrophil cytoplasm antibodies (ANCA) occur occasionally in rheumatoid arthritis (RA), but their incidence and clinical significance have been unclear. In this study we have investigated 58 patients with RA. In 22 patients the disease was inactive and the remaining 36 with active disease were further subdivided into those without clinical evidence of vasculitis (26), those with cutaneous vasculitis (8) and those with systemic vasculitis (2). ANCA were demonstrated by indirect immunofluorescence in 10 of the 58 patients (17%). While both perinuclear (pANCA) and cytoplasmic (cANCA) staining were detected, pANCA were more common (70%). Neutrophil-specific anti-nuclear antibodies (ANNA) were demonstrated in a further eight sera (14%) and ANA were detected on Hep-2 cells in 30 of the 58 sera (52%). ELISAs for the detection of anti-myeloperoxidase and anti-elastase antibodies were then established. Five sera with pANCA and five that contained ANNA were negative for both anti-myeloperoxidase and anti-elastase antibodies, suggesting other as yet unidentified cytoplasmic antigens as the target molecules. However, anti-myeloperoxidase or anti-elastase antibodies were found in four sera that had homogeneous or speckled ANA on both Hep-2 cells and neutrophils. One serum contained both antibodies. The presence of ANCA detected by indirect immunofluorescence or of anti-myeloperoxidase or anti-elastase antibodies in these patients with RA was not associated with disease activity nor with the demonstration of cutaneous vasculitis or renal disease (P NS). A possible association with systemic vasculitis remains to be confirmed. There is an incomplete correlation between indirect immunofluorescence patterns and antibody specificity in ELISA systems.
Full text
PDF

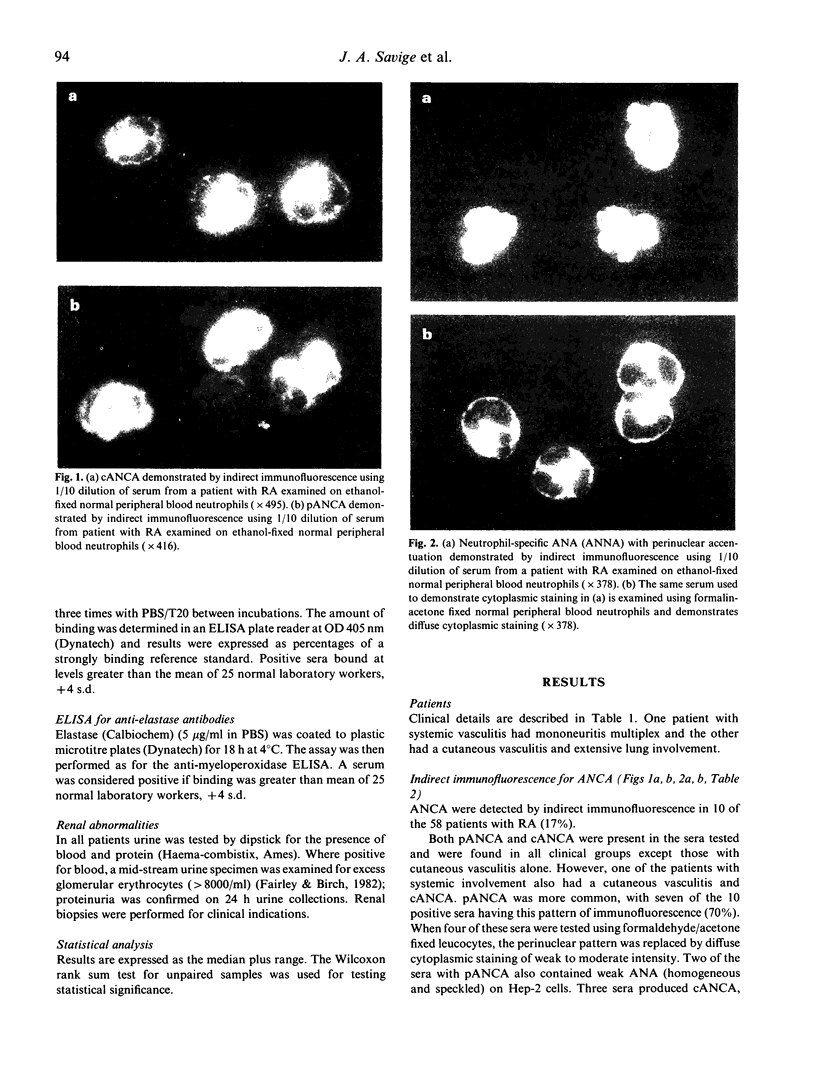
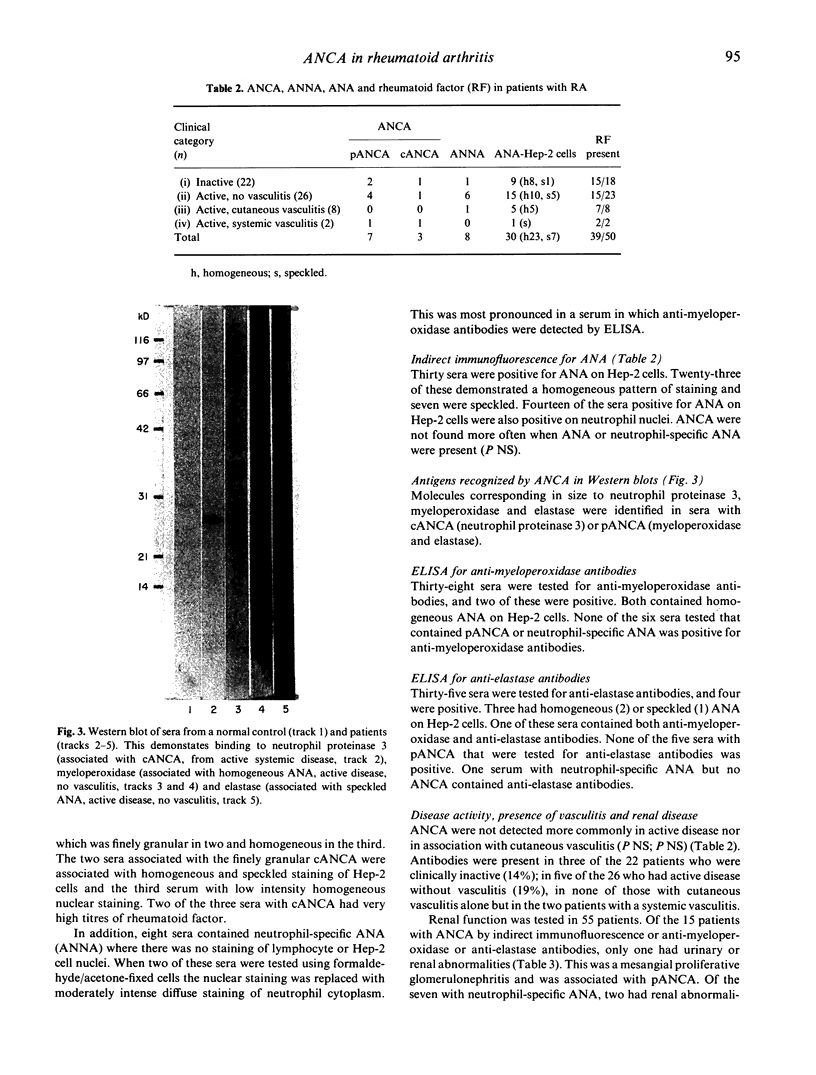



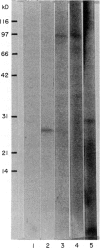
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- CALABRESI P., EDWARDS E. A., SCHILLING R. F. Fluorescent antiglobulin studies in leukopenic and related disorders. J Clin Invest. 1959 Nov;38:2091–2100. doi: 10.1172/JCI103987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn D. L., Schroeter A. L., McDuffie F. C. Cutaneous vessel immune deposits in rheumatoid arthritis. Arthritis Rheum. 1976 Jan-Feb;19(1):15–20. doi: 10.1002/art.1780190102. [DOI] [PubMed] [Google Scholar]
- Cunningham T. J., Tait B. D., Mathews J. D., Muirden K. D. Clinical rheumatoid vasculitis associated with the B8 DR3 phenotype. Rheumatol Int. 1982;2(3):137–139. doi: 10.1007/BF00541167. [DOI] [PubMed] [Google Scholar]
- Davies D. J., Moran J. E., Niall J. F., Ryan G. B. Segmental necrotising glomerulonephritis with antineutrophil antibody: possible arbovirus aetiology? 1982 Aug 28-Sep 4Br Med J (Clin Res Ed) 285(6342):606–606. doi: 10.1136/bmj.285.6342.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald K. J., Edwards R. L., McEvoy J. D. An ultrastructural study of the pathogenesis of tissue injury in limited Wegener's granulomatosis. Pathology. 1976 Apr;8(2):161–169. doi: 10.3109/00313027609094443. [DOI] [PubMed] [Google Scholar]
- Elling P. On the incidence of antinuclear factors in rheumatoid arthritis. A comparative study, with special reference to the reaction with polymorphonuclear granulocytes. Acta Rheumatol Scand. 1967;13(2):101–112. doi: 10.3109/rhe1.1967.13.issue-1-4.10. [DOI] [PubMed] [Google Scholar]
- Fairley K. F., Birch D. F. Hematuria: a simple method for identifying glomerular bleeding. Kidney Int. 1982 Jan;21(1):105–108. doi: 10.1038/ki.1982.16. [DOI] [PubMed] [Google Scholar]
- Falk R. J., Jennette J. C. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988 Jun 23;318(25):1651–1657. doi: 10.1056/NEJM198806233182504. [DOI] [PubMed] [Google Scholar]
- Falk R. J., Terrell R. S., Charles L. A., Jennette J. C. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4115–4119. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D. A., Stein J. L., Broder I. The extra-articular features of rheumatoid arthritis. A systematic analysis of 127 cases. Am J Med. 1973 Apr;54(4):445–452. doi: 10.1016/0002-9343(73)90040-5. [DOI] [PubMed] [Google Scholar]
- Hogg N., MacDonald S., Slusarenko M., Beverley P. C. Monoclonal antibodies specific for human monocytes, granulocytes and endothelium. Immunology. 1984 Dec;53(4):753–767. [PMC free article] [PubMed] [Google Scholar]
- Knowles D. M., 2nd, Tolidjian B., Marboe C., D'Agati V., Grimes M., Chess L. Monoclonal anti-human monocyte antibodies OKM1 and OKM5 possess distinctive tissue distributions including differential reactivity with vascular endothelium. J Immunol. 1984 May;132(5):2170–2173. [PubMed] [Google Scholar]
- Koderisch J., Andrassy K., Rasmussen N., Hartmann M., Tilgen W. "False-positive" anti-neutrophil cytoplasmic antibodies in HIV infection. Lancet. 1990 May 19;335(8699):1227–1228. doi: 10.1016/0140-6736(90)92755-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lüdemann J., Utecht B., Gross W. L. Anti-neutrophil cytoplasm antibodies in Wegener's granulomatosis recognize an elastinolytic enzyme. J Exp Med. 1990 Jan 1;171(1):357–362. doi: 10.1084/jem.171.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinching A. J., Lockwood C. M., Pussell B. A., Rees A. J., Sweny P., Evans D. J., Bowley N., Peters D. K. Wegener's granulomatosis: observations on 18 patients with severe renal disease. Q J Med. 1983 Autumn;52(208):435–460. [PubMed] [Google Scholar]
- Pinching A. J., Rees A. J., Pussell B. A., Lockwood C. M., Mitchison R. S., Peters D. K. Relapses in Wegener's granulomatosis: the role of infection. Br Med J. 1980 Sep 27;281(6244):836–838. doi: 10.1136/bmj.281.6244.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage C. O., Winearls C. G., Jones S., Marshall P. D., Lockwood C. M. Prospective study of radioimmunoassay for antibodies against neutrophil cytoplasm in diagnosis of systemic vasculitis. Lancet. 1987 Jun 20;1(8547):1389–1393. doi: 10.1016/s0140-6736(87)90591-5. [DOI] [PubMed] [Google Scholar]
- Savige J. A., Gallicchio M., Georgiou T., Davies D. J. Diverse target antigens recognized by circulating antibodies in anti-neutrophil cytoplasm antibody-associated renal vasculitides. Clin Exp Immunol. 1990 Nov;82(2):238–243. doi: 10.1111/j.1365-2249.1990.tb05433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savige J. A., Yeung S. P., Davies D. J., Ebeling P., Hunt D. H. Anti-neutrophil cytoplasmic antibodies associated with atrial myxoma. Am J Med. 1988 Nov;85(5):755–756. doi: 10.1016/s0002-9343(88)80275-4. [DOI] [PubMed] [Google Scholar]
- Scott D. G., Bacon P. A., Allen C., Elson C. J., Wallington T. IgG rheumatoid factor, complement and immune complexes in rheumatoid synovitis and vasculitis: comparative and serial studies during cytotoxic therapy. Clin Exp Immunol. 1981 Jan;43(1):54–63. [PMC free article] [PubMed] [Google Scholar]
- Scott D. G., Bacon P. A. Intravenous cyclophosphamide plus methylprednisolone in treatment of systemic rheumatoid vasculitis. Am J Med. 1984 Mar;76(3):377–384. doi: 10.1016/0002-9343(84)90654-5. [DOI] [PubMed] [Google Scholar]
- Scott D. G., Bacon P. A., Tribe C. R. Systemic rheumatoid vasculitis: a clinical and laboratory study of 50 cases. Medicine (Baltimore) 1981 Jul;60(4):288–297. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westgeest A. A., van Loon A. M., van der Logt J. T., van de Putte L. B., Boerbooms A. M. Antiperinuclear factor, a rheumatoid arthritis specific autoantibody: its relation to Epstein-Barr virus. J Rheumatol. 1989 May;16(5):626–630. [PubMed] [Google Scholar]
- van der Woude F. J., Rasmussen N., Lobatto S., Wiik A., Permin H., van Es L. A., van der Giessen M., van der Hem G. K., The T. H. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet. 1985 Feb 23;1(8426):425–429. doi: 10.1016/s0140-6736(85)91147-x. [DOI] [PubMed] [Google Scholar]