Abstract
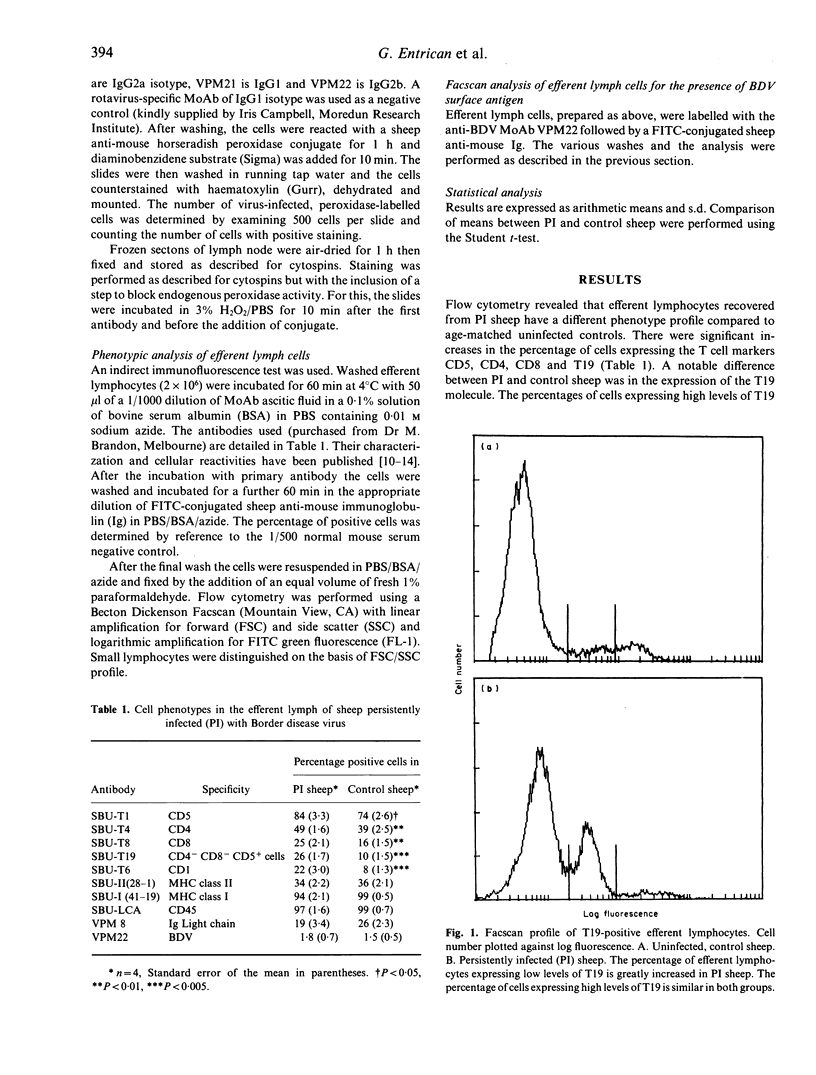
The prefemoral efferent lymphatics of sheep persistently infected (PI) with Border disease virus (BDV) were cannulated in order to study the effects of the virus on cells of the immune system. Efferent lymphocytes recovered from PI sheep were phenotyped using a panel of monoclonal antibodies (MoAb) specific for ovine cell-surface markers and compared to lymphocytes recovered from normal, healthy controls. PI sheep had an increased percentage of cells expressing the T cell-associated molecules CD5, CD4, CD8 and T19, also an increase in cells expressing CD1 and a population of cells expressing low levels of the T19 molecule which was not present in control sheep. The lymphocytes were examined for the presence of BDV using virus-specific MoAb. On average 8.5% of the efferent lymphocytes from PI sheep carried virus antigen. BDV antigen was also found in the mononuclear cells and connective tissue of lymph nodes indicating widespread virus dissemination within the lymphoid system of PI sheep.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow R. M., Gardiner A. C., Nettleton P. F. The pathology of a spontaneous and experimental mucosal disease-like syndrome in sheep recovered from clinical border disease. J Comp Pathol. 1983 Jul;93(3):451–461. doi: 10.1016/0021-9975(83)90032-4. [DOI] [PubMed] [Google Scholar]
- Bielefeldt Ohmann H. Double-immunolabeling systems for phenotyping of immune cells harboring bovine viral diarrhea virus. J Histochem Cytochem. 1987 Jun;35(6):627–633. doi: 10.1177/35.6.3033062. [DOI] [PubMed] [Google Scholar]
- Bielefeldt Ohmann H., Rønsholt L., Bloch B. Demonstration of bovine viral diarrhoea virus in peripheral blood mononuclear cells of persistently infected, clinically normal cattle. J Gen Virol. 1987 Jul;68(Pt 7):1971–1982. doi: 10.1099/0022-1317-68-7-1971. [DOI] [PubMed] [Google Scholar]
- Bonniwell M. A., Nettleton P. F., Gardiner A. C., Barlow R. M., Gilmour J. S. Border disease without nervous signs or fleece changes. Vet Rec. 1987 Mar 14;120(11):246–249. doi: 10.1136/vr.120.11.246. [DOI] [PubMed] [Google Scholar]
- Bujdoso R., Hopkins J., Dutia B. M., Young P., McConnell I. Characterization of sheep afferent lymph dendritic cells and their role in antigen carriage. J Exp Med. 1989 Oct 1;170(4):1285–1301. doi: 10.1084/jem.170.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrells C., Nettleton P. F., Reid H. W., Miller H. R., Hopkins J., McConnell I., Gorrell M. D., Brandon M. R. Lymphocyte subpopulations in the blood of sheep persistently infected with border disease virus. Clin Exp Immunol. 1989 Jun;76(3):446–451. [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Moennig V., Horzinek M. C. Recent advances in pestivirus research. J Gen Virol. 1989 Feb;70(Pt 2):253–266. doi: 10.1099/0022-1317-70-2-253. [DOI] [PubMed] [Google Scholar]
- Dutia B. M., Entrican G., Nettleton P. F. Cytopathic and non-cytopathic biotypes of border disease virus induce polypeptides of different molecular weight with common antigenic determinants. J Gen Virol. 1990 May;71(Pt 5):1227–1232. doi: 10.1099/0022-1317-71-5-1227. [DOI] [PubMed] [Google Scholar]
- Gardiner A. C., Nettleton P. F., Barlow R. M. Virology and immunology of a spontaneous and experimental mucosal disease-like syndrome in sheep recovered from clinical border disease. J Comp Pathol. 1983 Jul;93(3):463–469. doi: 10.1016/0021-9975(83)90033-6. [DOI] [PubMed] [Google Scholar]
- Gardiner A. C. The distribution and significance of border disease viral antigen in infected lambs and foetuses. J Comp Pathol. 1980 Oct;90(4):513–518. doi: 10.1016/0021-9975(80)90100-0. [DOI] [PubMed] [Google Scholar]
- Gardiner A. C., Zakarian B., Barlow R. M. Periarteritis in experimental border disease of sheep. III. Immunopathological observations. J Comp Pathol. 1980 Jul;90(3):469–474. doi: 10.1016/0021-9975(80)90016-x. [DOI] [PubMed] [Google Scholar]
- Gogolin-Ewens K. J., Mackay C. R., Mercer W. R., Brandon M. R. Sheep lymphocyte antigens (OLA). I. Major histocompatibility complex class I molecules. Immunology. 1985 Dec;56(4):717–723. [PMC free article] [PubMed] [Google Scholar]
- Hall J. G. A method for collecting lymph from the prefemoral lymph node of unanaesthetised sheep. Q J Exp Physiol Cogn Med Sci. 1967 Apr;52(2):200–205. doi: 10.1113/expphysiol.1967.sp001902. [DOI] [PubMed] [Google Scholar]
- Mackay C. R., Beya M. F., Matzinger P. Gamma/delta T cells express a unique surface molecule appearing late during thymic development. Eur J Immunol. 1989 Aug;19(8):1477–1483. doi: 10.1002/eji.1830190820. [DOI] [PubMed] [Google Scholar]
- Mackay C. R., Maddox J. F., Brandon M. R. Three distinct subpopulations of sheep T lymphocytes. Eur J Immunol. 1986 Jan;16(1):19–25. doi: 10.1002/eji.1830160105. [DOI] [PubMed] [Google Scholar]
- Mackay C. R., Maddox J. F., Gogolin-Ewens K. J., Brandon M. R. Characterization of two sheep lymphocyte differentiation antigens, SBU-T1 and SBU-T6. Immunology. 1985 Aug;55(4):729–737. [PMC free article] [PubMed] [Google Scholar]
- Mackay C. Sheep leukocyte molecules: a review of their distribution, structure and possible function. Vet Immunol Immunopathol. 1988 Jul;19(1):1–20. doi: 10.1016/0165-2427(88)90042-6. [DOI] [PubMed] [Google Scholar]
- Maddox J. F., Mackay C. R., Brandon M. R. Surface antigens, SBU-T4 and SBU-T8, of sheep T lymphocyte subsets defined by monoclonal antibodies. Immunology. 1985 Aug;55(4):739–748. [PMC free article] [PubMed] [Google Scholar]
- McClure S. J., Hein W. R. Functional characteristics of 197+ CD4- CD8- sheep T lymphocytes: expansion and differentiation of peripheral T cells. Immunol Cell Biol. 1989 Aug;67(Pt 4):223–231. doi: 10.1038/icb.1989.34. [DOI] [PubMed] [Google Scholar]
- Potts B. J., Berry L. J., Osburn B. I., Johnson K. P. Viral persistence and abnormalities of the central nervous system after congenital infection of sheep with border disease virus. J Infect Dis. 1985 Feb;151(2):337–343. doi: 10.1093/infdis/151.2.337. [DOI] [PubMed] [Google Scholar]
- Puri N. K., Mackay C. R., Brandon M. R. Sheep lymphocyte antigens (OLA). II. Major histocompatibility complex class II molecules. Immunology. 1985 Dec;56(4):725–733. [PMC free article] [PubMed] [Google Scholar]
- Qvist P., Houe H., Aasted B., Meyling A. Comparison of flow cytometry and virus isolation in cell culture for identification of cattle persistently infected with bovine viral diarrhea virus. J Clin Microbiol. 1991 Mar;29(3):660–661. doi: 10.1128/jcm.29.3.660-661.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra C. Detection of Border disease antigen in tissues of affected sheep and in cell cultures by immunofluorescence. Res Vet Sci. 1978 Nov;25(3):350–355. [PubMed] [Google Scholar]
- Westaway E. G., Brinton M. A., Gaidamovich SYa, Horzinek M. C., Igarashi A., Käriäinen L., Lvov D. K., Porterfield J. S., Russell P. K., Trent D. W. Togaviridae. Intervirology. 1985;24(3):125–139. doi: 10.1159/000149632. [DOI] [PubMed] [Google Scholar]
- Woldehiwet Z., Sharma R. Alterations in lymphocyte subpopulations in peripheral blood of sheep persistently infected with border disease virus. Vet Microbiol. 1990 Apr;22(2-3):153–160. doi: 10.1016/0378-1135(90)90102-2. [DOI] [PubMed] [Google Scholar]



