Abstract
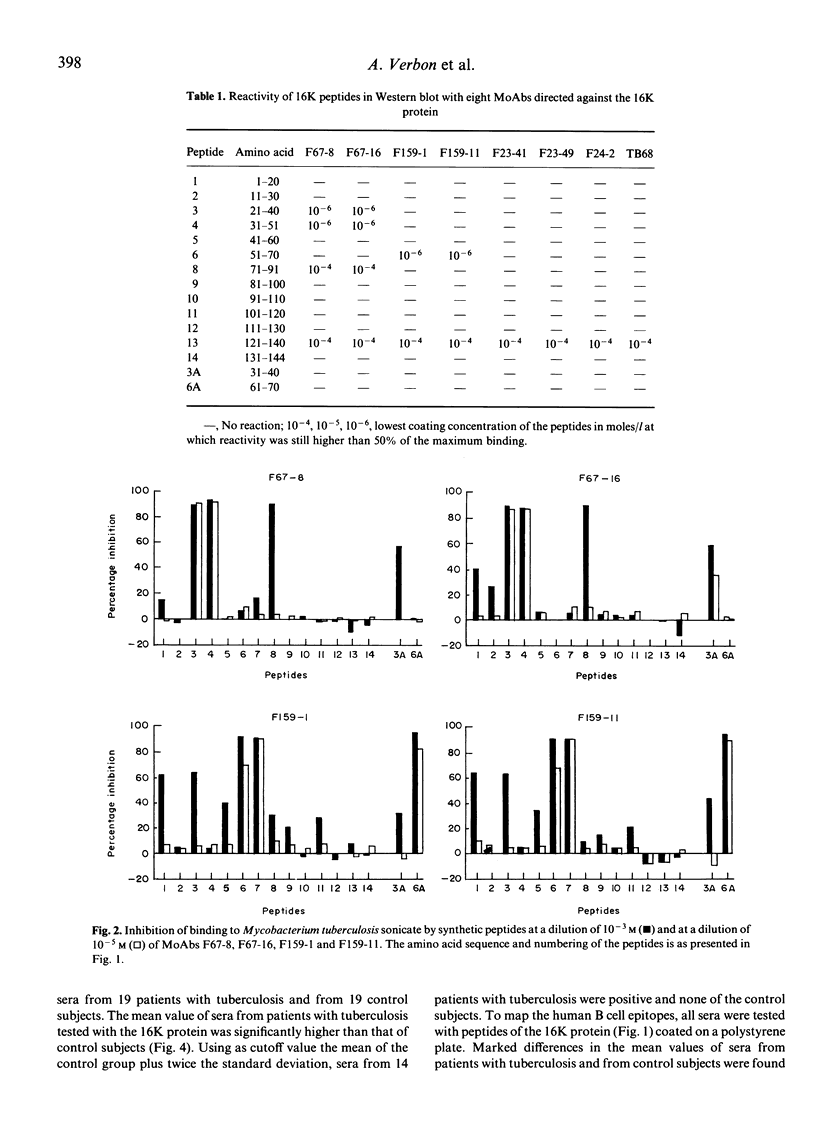
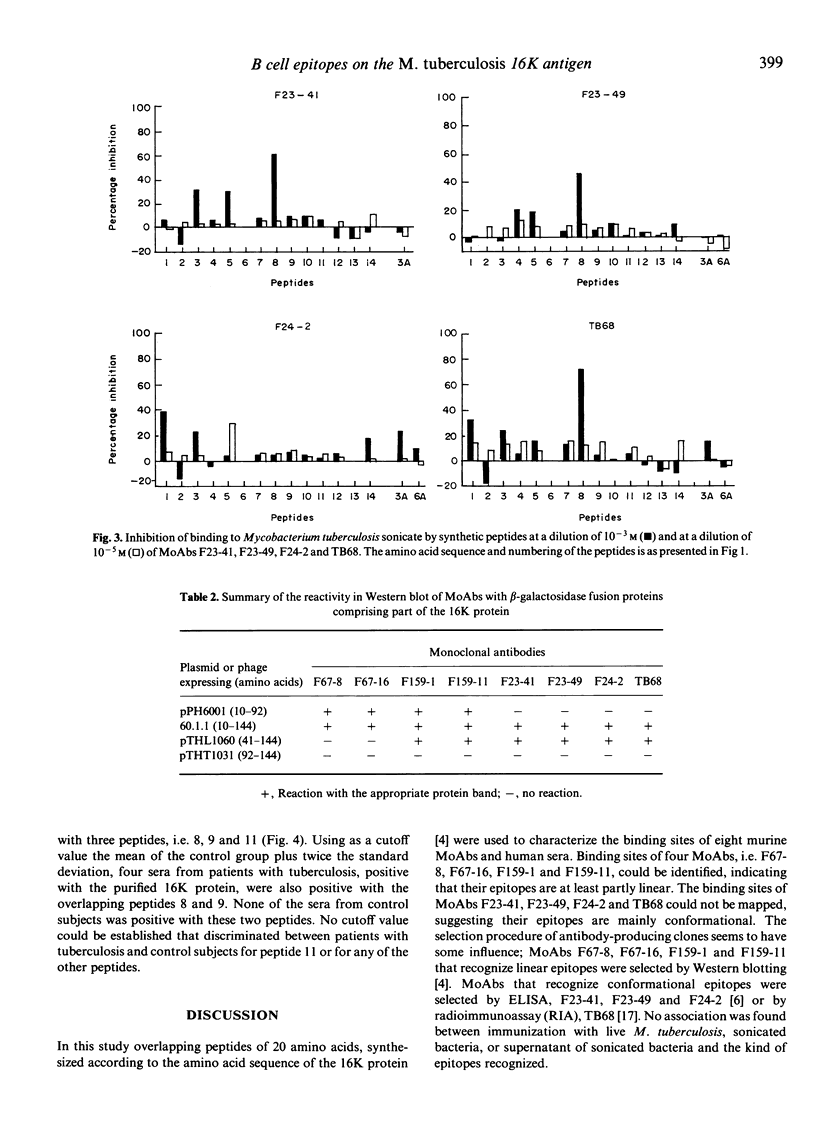
To characterize the antigenic parts of the 16K protein of Mycobacterium tuberculosis, overlapping peptides according to the amino acid sequence of the 16K protein were synthesized. In total, 14 peptides of 20 amino acids in length with an overlap of 10 amino acids and two additional decapeptides (amino acids 31-40 and 61-70) were tested with eight anti-16K MoAbs and human sera. The common recognition site of MoAbs F67-8 and F67-16 was LRPTFDTRLM (amino acids 31-40) and of MoAbs F159-1 and F159-11 DPDKDVDIMV (amino acids 61-70). However, for binding of the MoAbs to these peptides additional amino acids were required at either the N- or C-terminus, suggesting that some kind of conformation is required. The recognition sites of the MoAbs F23-41, F23-49, F24-2 and TB68 could not be identified using the peptides, indicating that the MoAbs only bound to conformational epitopes and not to peptides which may contain parts of these epitopes. The MoAbs bound to beta-galactosidase fusion proteins comprising parts of the 16K protein, indicating that some kind of native conformation is present on the recombinant proteins. Sera from 14 of 19 patients with tuberculosis and none from 19 controls reacted with the purified 16K protein. Sera from four of these 14 patients reacted with two overlapping peptides (amino acids 71-100). Apparently, antibodies in patients' sera against the 16K protein are predominantly directed against conformational epitopes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atassi M. Z., Saplin B. J. Immunochemistry of sperm whale myoglobin. I. The specific interaction of some tryptic peptides and of peptides containing all the reactive regions of the antigen. Biochemistry. 1968 Feb;7(2):688–698. doi: 10.1021/bi00842a026. [DOI] [PubMed] [Google Scholar]
- Berzofsky J. A. Intrinsic and extrinsic factors in protein antigenic structure. Science. 1985 Sep 6;229(4717):932–940. doi: 10.1126/science.2410982. [DOI] [PubMed] [Google Scholar]
- Brudney K., Dobkin J. Resurgent tuberculosis in New York City. Human immunodeficiency virus, homelessness, and the decline of tuberculosis control programs. Am Rev Respir Dis. 1991 Oct;144(4):745–749. doi: 10.1164/ajrccm/144.4.745. [DOI] [PubMed] [Google Scholar]
- Chandramuki A., Bothamley G. H., Brennan P. J., Ivanyi J. Levels of antibody to defined antigens of Mycobacterium tuberculosis in tuberculous meningitis. J Clin Microbiol. 1989 May;27(5):821–825. doi: 10.1128/jcm.27.5.821-825.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates A. R., Hewitt J., Allen B. W., Ivanyi J., Mitchison D. A. Antigenic diversity of Mycobacterium tuberculosis and Mycobacterium bovis detected by means of monoclonal antibodies. Lancet. 1981 Jul 25;2(8239):167–169. doi: 10.1016/s0140-6736(81)90355-x. [DOI] [PubMed] [Google Scholar]
- Ivanyi J., Bothamley G. H., Jackett P. S. Immunodiagnostic assays for tuberculosis and leprosy. Br Med Bull. 1988 Jul;44(3):635–649. doi: 10.1093/oxfordjournals.bmb.a072273. [DOI] [PubMed] [Google Scholar]
- Jackett P. S., Bothamley G. H., Batra H. V., Mistry A., Young D. B., Ivanyi J. Specificity of antibodies to immunodominant mycobacterial antigens in pulmonary tuberculosis. J Clin Microbiol. 1988 Nov;26(11):2313–2318. doi: 10.1128/jcm.26.11.2313-2318.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W., Sander C. On the use of sequence homologies to predict protein structure: identical pentapeptides can have completely different conformations. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1075–1078. doi: 10.1073/pnas.81.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatser P. R., De Wit M. Y., Kolk A. H., Hartskeerl R. A. Characterization of murine B-cell epitopes on the Mycobacterium leprae proline-rich antigen by use of synthetic peptides. Infect Immun. 1991 Jan;59(1):433–436. doi: 10.1128/iai.59.1.433-436.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolk A. H., Evers R., Groothuis D. G., Gilis H., Kuijper S. Production and characterization of monoclonal antibodies against specific serotypes of Mycobacterium avium and the Mycobacterium avium-Mycobacterium intracellulare-Mycobacterium scrofulaceum complex. Infect Immun. 1989 Aug;57(8):2514–2521. doi: 10.1128/iai.57.8.2514-2521.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolk A. H., Ho M. L., Klatser P. R., Eggelte T. A., Kuijper S., de Jonge S., van Leeuwen J. Production and characterization of monoclonal antibodies to Mycobacterium tuberculosis, M. bovis (BCG) and M. leprae. Clin Exp Immunol. 1984 Dec;58(3):511–521. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lal R. B., Rudolph D. L., Lairmore M. D., Khabbaz R. F., Garfield M., Coligan J. E., Folks T. M. Serologic discrimination of human T cell lymphotropic virus infection by using a synthetic peptide-based enzyme immunoassay. J Infect Dis. 1991 Jan;163(1):41–46. doi: 10.1093/infdis/163.1.41. [DOI] [PubMed] [Google Scholar]
- Minden P., Kelleher P. J., Freed J. H., Nielsen L. D., Brennan P. J., McPheron L., McClatchy J. K. Immunological evaluation of a component isolated from Mycobacterium bovis BCG with a monoclonal antibody to M. bovis BCG. Infect Immun. 1984 Nov;46(2):519–525. doi: 10.1128/iai.46.2.519-525.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C. J., Styblo K., Rouillon A. Tuberculosis in developing countries: burden, intervention and cost. Bull Int Union Tuberc Lung Dis. 1990 Mar;65(1):6–24. [PubMed] [Google Scholar]
- Norrby E., Mufson M. A., Alexander H., Houghten R. A., Lerner R. A. Site-directed serology with synthetic peptides representing the large glycoprotein G of respiratory syncytial virus. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6572–6576. doi: 10.1073/pnas.84.18.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Results of a World Health Organization-sponsored workshop to characterize antigens recognized by mycobacterium-specific monoclonal antibodies. Infect Immun. 1986 Feb;51(2):718–720. doi: 10.1128/iai.51.2.718-720.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöningh R., Verstijnen C. P., Kuijper S., Kolk A. H. Enzyme immunoassay for identification of heat-killed mycobacteria belonging to the Mycobacterium tuberculosis and Mycobacterium avium complexes and derived from early cultures. J Clin Microbiol. 1990 Apr;28(4):708–713. doi: 10.1128/jcm.28.4.708-713.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Regenmortel M. H. Structural and functional approaches to the study of protein antigenicity. Immunol Today. 1989 Aug;10(8):266–272. doi: 10.1016/0167-5699(89)90140-0. [DOI] [PubMed] [Google Scholar]
- Verbon A., Hartskeerl R. A., Kolk A. H. Murine and human B cell epitope mapping of the Mycobacterium tuberculosis 10-kD heat shock protein using overlapping peptides. Clin Exp Immunol. 1991 Oct;86(1):6–12. doi: 10.1111/j.1365-2249.1991.tb05765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbon A., Hartskeerl R. A., Schuitema A., Kolk A. H., Young D. B., Lathigra R. The 14,000-molecular-weight antigen of Mycobacterium tuberculosis is related to the alpha-crystallin family of low-molecular-weight heat shock proteins. J Bacteriol. 1992 Feb;174(4):1352–1359. doi: 10.1128/jb.174.4.1352-1359.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbon A., Kuijper S., Jansen H. M., Speelman P., Kolk A. H. Antigens in culture supernatant of Mycobacterium tuberculosis: epitopes defined by monoclonal and human antibodies. J Gen Microbiol. 1990 May;136(5):955–964. doi: 10.1099/00221287-136-5-955. [DOI] [PubMed] [Google Scholar]
- Verstijnen C. P., Ly H. M., Polman K., Richter C., Smits S. P., Maselle S. Y., Peerbooms P., Rienthong D., Montreewasuwat N., Koanjanart S. Enzyme-linked immunosorbent assay using monoclonal antibodies for identification of mycobacteria from early cultures. J Clin Microbiol. 1991 Jul;29(7):1372–1375. doi: 10.1128/jcm.29.7.1372-1375.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vordemeier H. M., Harris D. P., Roman E., Lathigra R., Moreno C., Ivanyi J. Identification of T cell stimulatory peptides from the 38-kDa protein of Mycobacterium tuberculosis. J Immunol. 1991 Aug 1;147(3):1023–1029. [PubMed] [Google Scholar]
- Wilson I. A., Haft D. H., Getzoff E. D., Tainer J. A., Lerner R. A., Brenner S. Identical short peptide sequences in unrelated proteins can have different conformations: a testing ground for theories of immune recognition. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5255–5259. doi: 10.1073/pnas.82.16.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. B., Kaufmann S. H., Hermans P. W., Thole J. E. Mycobacterial protein antigens: a compilation. Mol Microbiol. 1992 Jan;6(2):133–145. doi: 10.1111/j.1365-2958.1992.tb01994.x. [DOI] [PubMed] [Google Scholar]
- Young R. A., Bloom B. R., Grosskinsky C. M., Ivanyi J., Thomas D., Davis R. W. Dissection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2583–2587. doi: 10.1073/pnas.82.9.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabeau M., Stanley K. K. Enhanced expression of cro-beta-galactosidase fusion proteins under the control of the PR promoter of bacteriophage lambda. EMBO J. 1982;1(10):1217–1224. doi: 10.1002/j.1460-2075.1982.tb00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


