Abstract
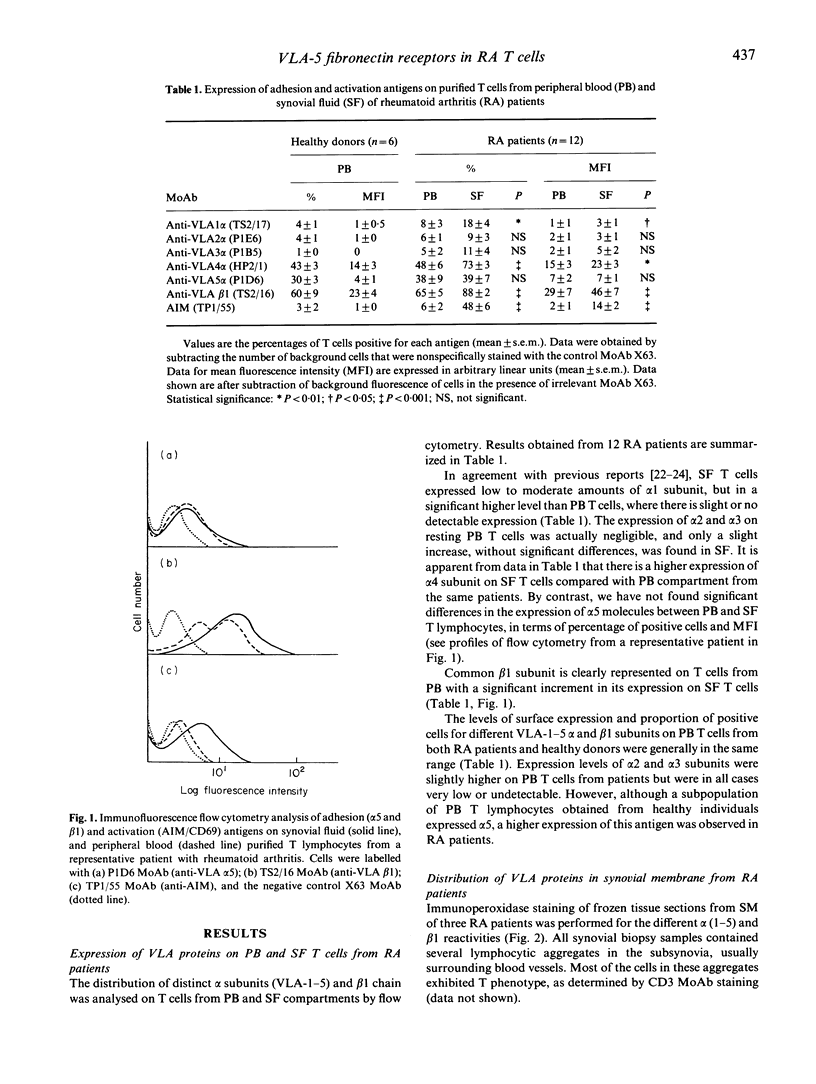
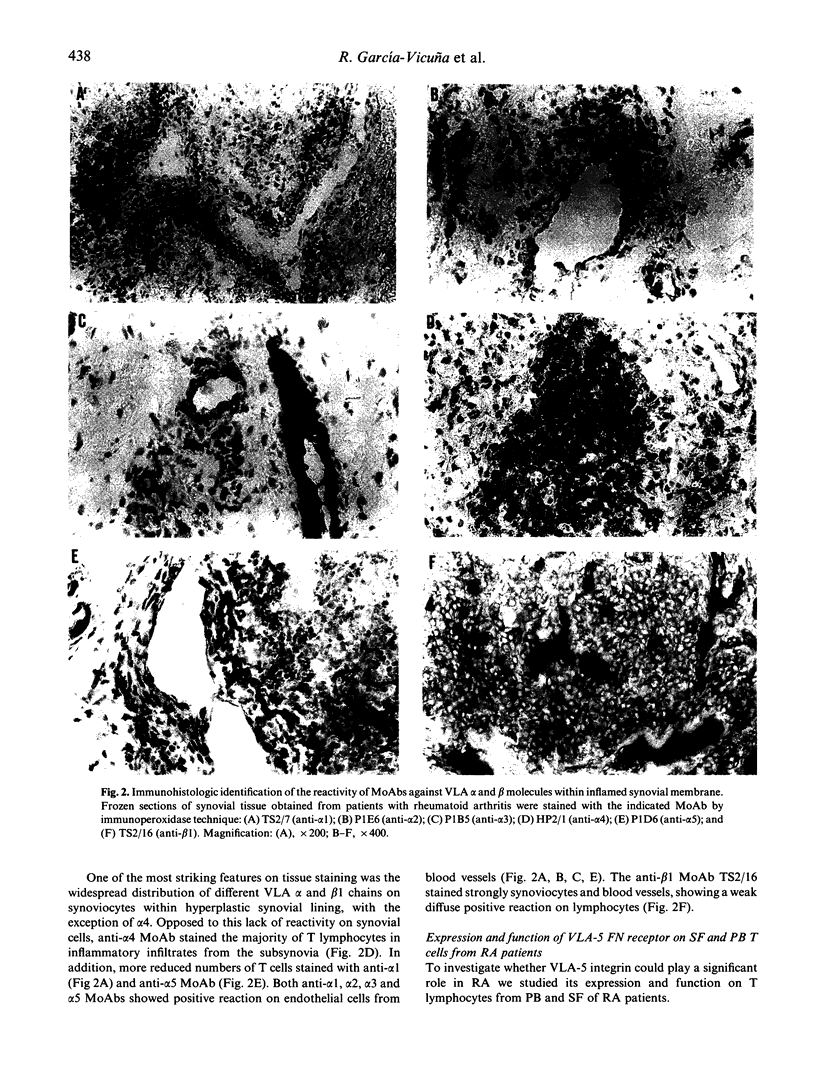
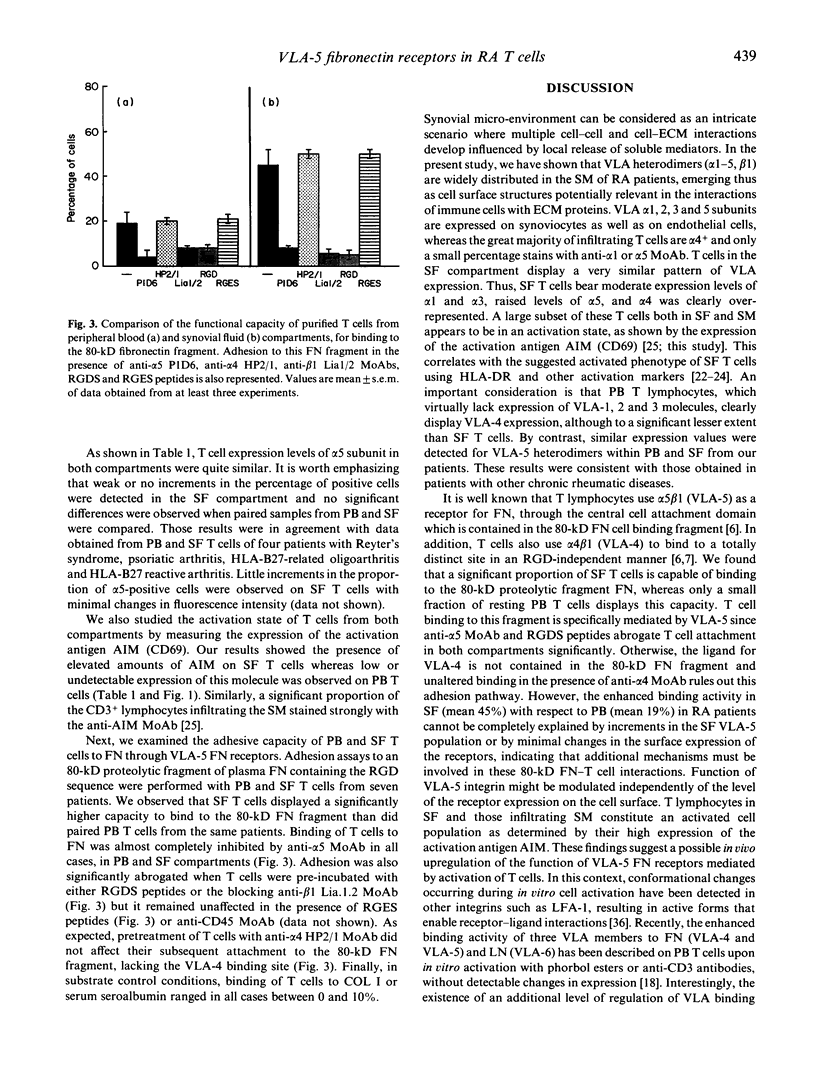
Adhesion of T cells to extracellular matrix (ECM) proteins through VLA integrin receptors is crucial for lymphocyte trafficking, tissue localization and inflammatory function. We have investigated the expression of different VLA integrins (VLA-1-5) on peripheral blood (PB) and synovial fluid (SF) T lymphocytes from patients with rheumatoid arthritis (RA). Their expression on different cell types from synovial membrane (SM) is also reported. The role of VLA-4 fibronectin (FN) receptors in the interaction of activated SF T cells from RA patients with a 38-kD fragment of FN has been previously demonstrated. Here we have focused functional studies on VLA-5 as an alternative FN receptor for RA T cells. A significant higher proportion of SF T cells were able to bind to an 80-kD fragment of FN, containing the Arg-Gly-Asp (RGD) cell binding site, compared with PB T cells. This attachment was almost completely inhibited by anti-VLA-5 MoAbs as well as by RGD peptides. This enhanced capability by SF T cells appears to be independent of the level of the surface expression of the receptor and correlates better with their activation state as determined by the expression of the activation molecule AIM (CD69). The evidence for the expression of VLA heterodimers on both SF and SM cells from RA patients suggests the possible implication of ECM proteins in mediating and perpetuating inflammation in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C., Watt F. M. Changes in keratinocyte adhesion during terminal differentiation: reduction in fibronectin binding precedes alpha 5 beta 1 integrin loss from the cell surface. Cell. 1990 Oct 19;63(2):425–435. doi: 10.1016/0092-8674(90)90175-e. [DOI] [PubMed] [Google Scholar]
- Akbar A. N., Terry L., Timms A., Beverley P. C., Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988 Apr 1;140(7):2171–2178. [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Bednarczyk J. L., McIntyre B. W. A monoclonal antibody to VLA-4 alpha-chain (CDw49d) induces homotypic lymphocyte aggregation. J Immunol. 1990 Feb 1;144(3):777–784. [PubMed] [Google Scholar]
- Campanero M. R., Pulido R., Ursa M. A., Rodríguez-Moya M., de Landázuri M. O., Sánchez-Madrid F. An alternative leukocyte homotypic adhesion mechanism, LFA-1/ICAM-1-independent, triggered through the human VLA-4 integrin. J Cell Biol. 1990 Jun;110(6):2157–2165. doi: 10.1083/jcb.110.6.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebrián M., Yagüe E., Rincón M., López-Botet M., de Landázuri M. O., Sánchez-Madrid F. Triggering of T cell proliferation through AIM, an activation inducer molecule expressed on activated human lymphocytes. J Exp Med. 1988 Nov 1;168(5):1621–1637. doi: 10.1084/jem.168.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charo I. F., Nannizzi L., Smith J. W., Cheresh D. A. The vitronectin receptor alpha v beta 3 binds fibronectin and acts in concert with alpha 5 beta 1 in promoting cellular attachment and spreading on fibronectin. J Cell Biol. 1990 Dec;111(6 Pt 1):2795–2800. doi: 10.1083/jcb.111.6.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayberger C., Krensky A. M., McIntyre B. W., Koller T. D., Parham P., Brodsky F., Linn D. J., Evans E. L. Identification and characterization of two novel lymphocyte function-associated antigens, L24 and L25. J Immunol. 1987 Mar 1;138(5):1510–1514. [PubMed] [Google Scholar]
- Clemmensen I., Andersen R. B. Different molecular forms of fibronectin in rheumatoid synovial fluid. Arthritis Rheum. 1982 Jan;25(1):25–31. doi: 10.1002/art.1780250104. [DOI] [PubMed] [Google Scholar]
- Cush J. J., Lipsky P. E. Phenotypic analysis of synovial tissue and peripheral blood lymphocytes isolated from patients with rheumatoid arthritis. Arthritis Rheum. 1988 Oct;31(10):1230–1238. doi: 10.1002/art.1780311003. [DOI] [PubMed] [Google Scholar]
- Davis L. S., Oppenheimer-Marks N., Bednarczyk J. L., McIntyre B. W., Lipsky P. E. Fibronectin promotes proliferation of naive and memory T cells by signaling through both the VLA-4 and VLA-5 integrin molecules. J Immunol. 1990 Aug 1;145(3):785–793. [PubMed] [Google Scholar]
- Dedhar S., Gray V. Isolation of a novel integrin receptor mediating Arg-Gly-Asp-directed cell adhesion to fibronectin and type I collagen from human neuroblastoma cells. Association of a novel beta 1-related subunit with alpha v. J Cell Biol. 1990 Jun;110(6):2185–2193. doi: 10.1083/jcb.110.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990 Feb 23;60(4):577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Emery P., Gentry K. C., Mackay I. R., Muirden K. D., Rowley M. Deficiency of the suppressor inducer subset of T lymphocytes in rheumatoid arthritis. Arthritis Rheum. 1987 Aug;30(8):849–856. doi: 10.1002/art.1780300802. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Hynes R. O. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor alpha 4 beta 1. Cell. 1990 Jan 12;60(1):53–61. doi: 10.1016/0092-8674(90)90715-q. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Glass D., Coblyn J. S., Jacobson J. G. Very late activation antigens on rheumatoid synovial fluid T lymphocytes. Association with stages of T cell activation. J Clin Invest. 1986 Sep;78(3):696–702. doi: 10.1172/JCI112629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M. E., Sanchez-Madrid F., Flotte T. J., Krensky A. M., Burakoff S. J., Bhan A. K., Springer T. A., Strominger J. L. Glycoproteins of 210,000 and 130,000 m.w. on activated T cells: cell distribution and antigenic relation to components on resting cells and T cell lines. J Immunol. 1984 Jun;132(6):3011–3018. [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Herbert K. E., Coppock J. S., Griffiths A. M., Williams A., Robinson M. W., Scott D. L. Fibronectin and immune complexes in rheumatic diseases. Ann Rheum Dis. 1987 Oct;46(10):734–740. doi: 10.1136/ard.46.10.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Marcantonio E. E., Stepp M. A., Urry L. A., Yee G. H. Integrin heterodimer and receptor complexity in avian and mammalian cells. J Cell Biol. 1989 Jul;109(1):409–420. doi: 10.1083/jcb.109.1.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keizer G. D., Borst J., Figdor C. G., Spits H., Miedema F., Terhorst C., De Vries J. E. Biochemical and functional characteristics of the human leukocyte membrane antigen family LFA-1, Mo-1 and p150,95. Eur J Immunol. 1985 Nov;15(11):1142–1148. doi: 10.1002/eji.1830151114. [DOI] [PubMed] [Google Scholar]
- Laffon A., Sánchez-Madrid F., Ortíz de Landázuri M., Jiménez Cuesta A., Ariza A., Ossorio C., Sabando P. Very late activation antigen on synovial fluid T cells from patients with rheumatoid arthritis and other rheumatic diseases. Arthritis Rheum. 1989 Apr;32(4):386–392. doi: 10.1002/anr.1780320405. [DOI] [PubMed] [Google Scholar]
- Laffón A., García-Vicuña R., Humbría A., Postigo A. A., Corbí A. L., de Landázuri M. O., Sánchez-Madrid F. Upregulated expression and function of VLA-4 fibronectin receptors on human activated T cells in rheumatoid arthritis. J Clin Invest. 1991 Aug;88(2):546–552. doi: 10.1172/JCI115338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky H. P., Bauer K., Pope R. M. Increased helper inducer and decreased suppressor inducer phenotypes in the rheumatoid joint. Arthritis Rheum. 1988 Jan;31(1):52–59. doi: 10.1002/art.1780310108. [DOI] [PubMed] [Google Scholar]
- Matsuyama T., Yamada A., Kay J., Yamada K. M., Akiyama S. K., Schlossman S. F., Morimoto C. Activation of CD4 cells by fibronectin and anti-CD3 antibody. A synergistic effect mediated by the VLA-5 fibronectin receptor complex. J Exp Med. 1989 Oct 1;170(4):1133–1148. doi: 10.1084/jem.170.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzalis C., Kingsley G., Murphy J., Panayi G. Abnormal distribution of the helper-inducer and suppressor-inducer T-lymphocyte subsets in the rheumatoid joint. Clin Immunol Immunopathol. 1987 Nov;45(2):252–258. doi: 10.1016/0090-1229(87)90040-7. [DOI] [PubMed] [Google Scholar]
- Pulido R., Cebrián M., Acevedo A., de Landázuri M. O., Sánchez-Madrid F. Comparative biochemical and tissue distribution study of four distinct CD45 antigen specificities. J Immunol. 1988 Jun 1;140(11):3851–3857. [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz B. R., Wayner E. A., Carlos T. M., Ochs H. D., Harlan J. M. Identification of surface proteins mediating adherence of CD11/CD18-deficient lymphoblastoid cells to cultured human endothelium. J Clin Invest. 1990 Jun;85(6):2019–2022. doi: 10.1172/JCI114668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D. L., Wainwright A. C., Walton K. W., Williamson N. Significance of fibronectin in rheumatoid arthritis and osteoarthrosis. Ann Rheum Dis. 1981 Apr;40(2):142–153. doi: 10.1136/ard.40.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Van Seventer G. A., Horgan K. J., Shaw S. Regulated expression and binding of three VLA (beta 1) integrin receptors on T cells. Nature. 1990 May 17;345(6272):250–253. doi: 10.1038/345250a0. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., van Seventer G. A., Horgan K. J., Shaw S. Costimulation of proliferative responses of resting CD4+ T cells by the interaction of VLA-4 and VLA-5 with fibronectin or VLA-6 with laminin. J Immunol. 1990 Jul 1;145(1):59–67. [PubMed] [Google Scholar]
- Spits H., Yssel H., Leeuwenberg J., De Vries J. E. Antigen-specific cytotoxic T cell and antigen-specific proliferating T cell clones can be induced to cytolytic activity by monoclonal antibodies against T3. Eur J Immunol. 1985 Jan;15(1):88–91. doi: 10.1002/eji.1830150117. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Sánchez-Madrid F., De Landázuri M. O., Morago G., Cebrián M., Acevedo A., Bernabeu C. VLA-3: a novel polypeptide association within the VLA molecular complex: cell distribution and biochemical characterization. Eur J Immunol. 1986 Nov;16(11):1343–1349. doi: 10.1002/eji.1830161106. [DOI] [PubMed] [Google Scholar]
- Takada Y., Wayner E. A., Carter W. G., Hemler M. E. Extracellular matrix receptors, ECMRII and ECMRI, for collagen and fibronectin correspond to VLA-2 and VLA-3 in the VLA family of heterodimers. J Cell Biochem. 1988 Aug;37(4):385–393. doi: 10.1002/jcb.240370406. [DOI] [PubMed] [Google Scholar]
- Vogel B. E., Tarone G., Giancotti F. G., Gailit J., Ruoslahti E. A novel fibronectin receptor with an unexpected subunit composition (alpha v beta 1). J Biol Chem. 1990 Apr 15;265(11):5934–5937. [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G., Piotrowicz R. S., Kunicki T. J. The function of multiple extracellular matrix receptors in mediating cell adhesion to extracellular matrix: preparation of monoclonal antibodies to the fibronectin receptor that specifically inhibit cell adhesion to fibronectin and react with platelet glycoproteins Ic-IIa. J Cell Biol. 1988 Nov;107(5):1881–1891. doi: 10.1083/jcb.107.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Garcia-Pardo A., Humphries M. J., McDonald J. A., Carter W. G. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989 Sep;109(3):1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]