Abstract
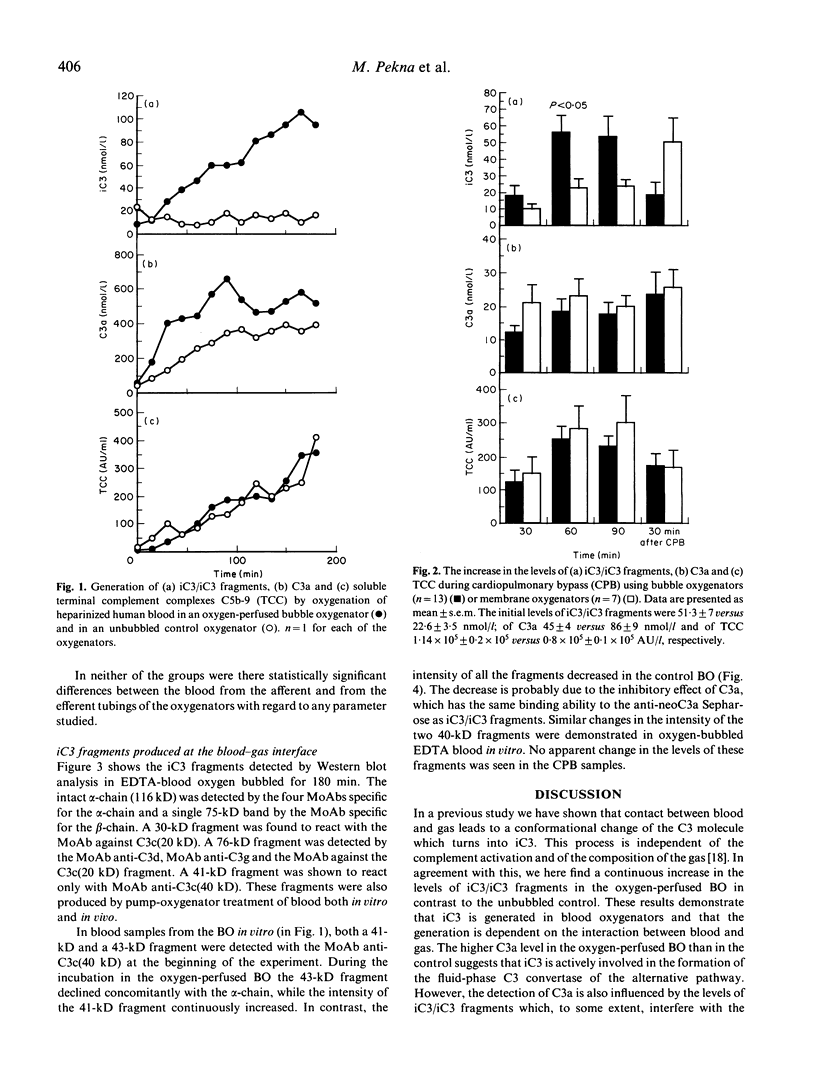
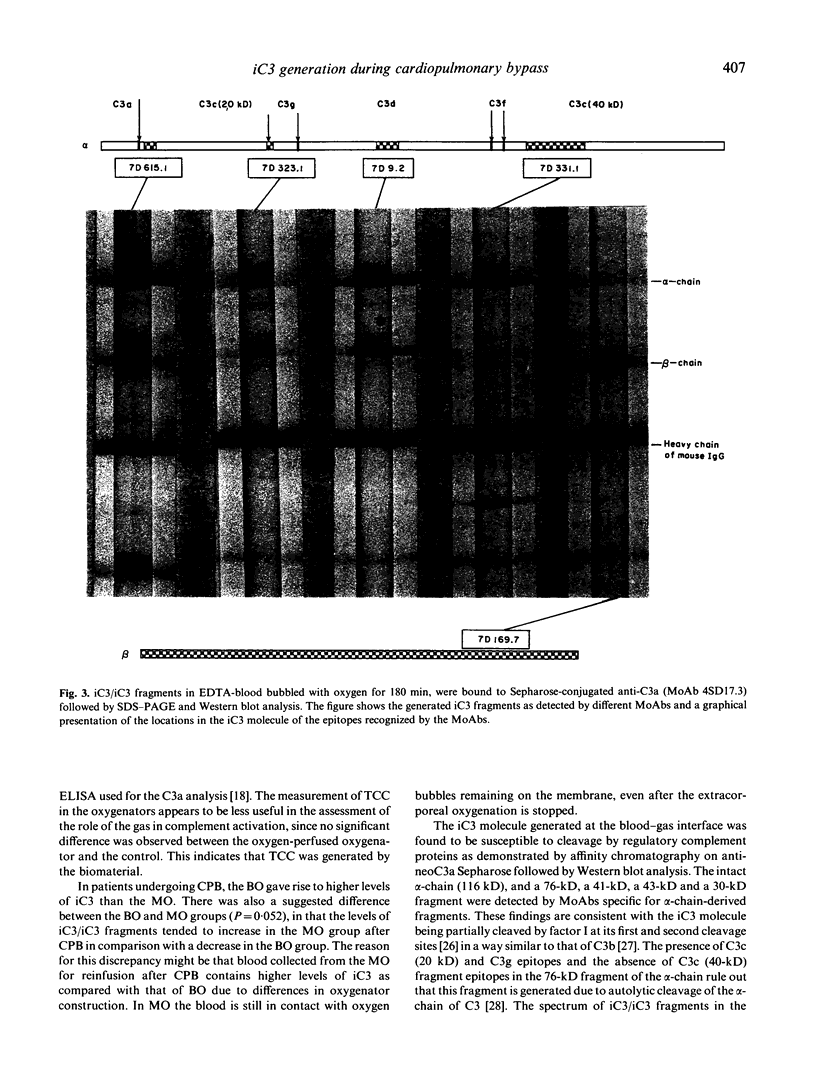
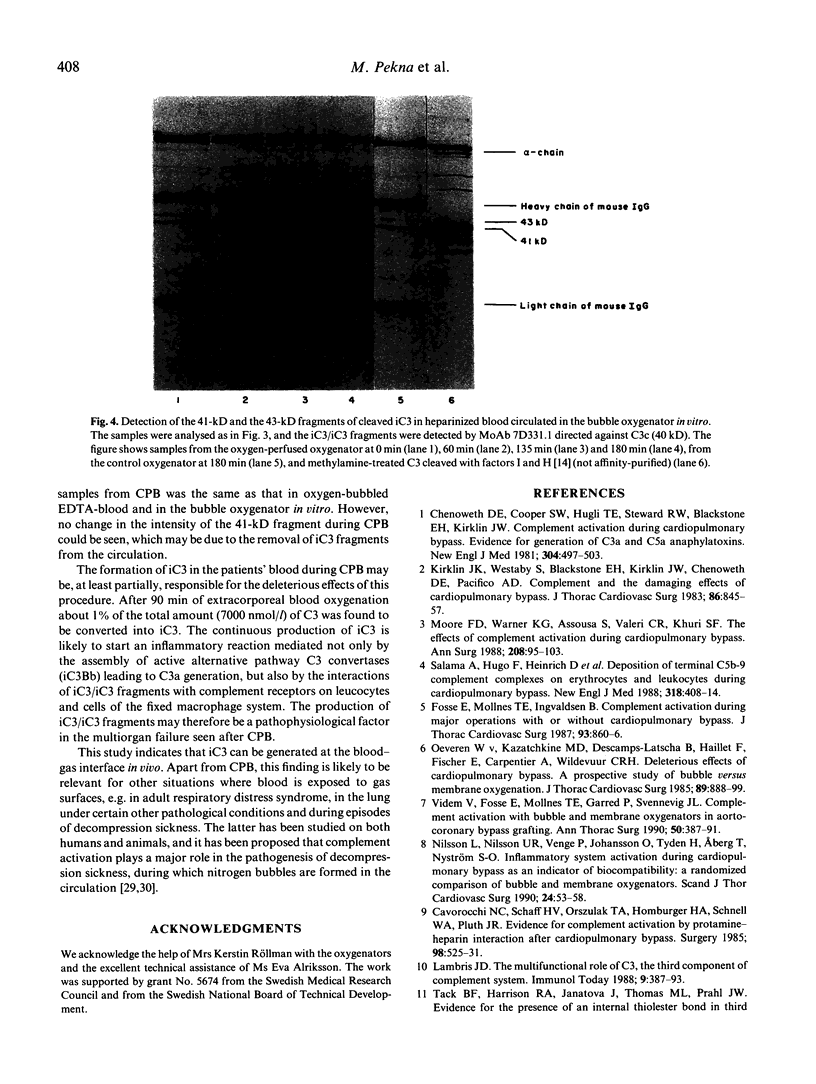
Earlier we have shown that iC3 is generated at the blood-gas interface in vitro and that the generation of this molecule is independent of complement activation and the composition of the gas. In order to investigate whether iC3 is also generated during cardiopulmonary bypass where blood comes into contact with oxygen bubbles, two bubble oxygenators were incubated at 37 degrees C with human heparinized blood. A continuous increase in the level of iC3 was shown in the oxygen-perfused bubble oxygenator (up to 100 nmol/l after 180 min) in contrast to the unbubbled control. Similarly, in plasma drawn from patients undergoing cardiopulmonary bypass using either bubble or membrane oxygenators, the levels of iC3 were shown to increase continuously during the operation. Furthermore, this form of C3 was found to be susceptible to cleavage by factor I. The formation of iC3 at the blood-gas interface in vivo could be a mechanism by which gas bubbles induce clinical manifestations associated with complement activation, e.g. during cardiopulmonary bypass, adult respiratory distress syndrome and decompression sickness.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chenoweth D. E., Cooper S. W., Hugli T. E., Stewart R. W., Blackstone E. H., Kirklin J. W. Complement activation during cardiopulmonary bypass: evidence for generation of C3a and C5a anaphylatoxins. N Engl J Med. 1981 Feb 26;304(9):497–503. doi: 10.1056/NEJM198102263040901. [DOI] [PubMed] [Google Scholar]
- Fosse E., Mollnes T. E., Ingvaldsen B. Complement activation during major operations with or without cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1987 Jun;93(6):860–866. [PubMed] [Google Scholar]
- Harrison R. A., Lachmann P. J. The physiological breakdown of the third component of human complement. Mol Immunol. 1980 Jan;17(1):9–20. doi: 10.1016/0161-5890(80)90119-4. [DOI] [PubMed] [Google Scholar]
- Isenman D. E., Kells D. I., Cooper N. R., Müller-Eberhard H. J., Pangburn M. K. Nucleophilic modification of human complement protein C3: correlation of conformational changes with acquisition of C3b-like functional properties. Biochemistry. 1981 Jul 21;20(15):4458–4467. doi: 10.1021/bi00518a034. [DOI] [PubMed] [Google Scholar]
- Kirklin J. K., Westaby S., Blackstone E. H., Kirklin J. W., Chenoweth D. E., Pacifico A. D. Complement and the damaging effects of cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1983 Dec;86(6):845–857. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Jr, Hairston P. Structural effects on blood proteins at the gas-blood interface. Fed Proc. 1971 Sep-Oct;30(5):1615–1622. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Mollnes T. E., Lea T., Frøland S. S., Harboe M. Quantification of the terminal complement complex in human plasma by an enzyme-linked immunosorbent assay based on monoclonal antibodies against a neoantigen of the complex. Scand J Immunol. 1985 Aug;22(2):197–202. doi: 10.1111/j.1365-3083.1985.tb01871.x. [DOI] [PubMed] [Google Scholar]
- Moore F. D., Jr, Warner K. G., Assousa S., Valeri C. R., Khuri S. F. The effects of complement activation during cardiopulmonary bypass. Attenuation by hypothermia, heparin, and hemodilution. Ann Surg. 1988 Jul;208(1):95–103. doi: 10.1097/00000658-198807000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson Ekdahl K., Nilsson B., Pekna M., Nilsson U. R. Generation of iC3 at the interface between blood and gas. Scand J Immunol. 1992 Jan;35(1):85–91. doi: 10.1111/j.1365-3083.1992.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Nilsson B., Grossberger D., Nilsson Ekdahl K., Riegert P., Becherer D. J., Nilsson U. R., Lambris J. D. Conformational differences between surface-bound and fluid-phase complement-component-C3 fragments. Epitope mapping by cDNA expression. Biochem J. 1992 Mar 15;282(Pt 3):715–721. doi: 10.1042/bj2820715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson B., Nilsson Ekdahl K., Avila D., Nilsson U. R., Lambris J. D. Neoantigens in complement component C3 as detected by monoclonal antibodies. Mapping of the recognized epitopes by synthetic peptides. Biochem J. 1990 May 15;268(1):55–61. doi: 10.1042/bj2680055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson B., Nilsson U. R. An assessment of the extent of antigenic analogy between physiologically bound C3 and C3 denatured by sodium dodecyl sulphate. Scand J Immunol. 1985 Dec;22(6):703–710. doi: 10.1111/j.1365-3083.1985.tb01933.x. [DOI] [PubMed] [Google Scholar]
- Nilsson B., Nilsson U. R. Antigens of complement factor C3 involved in the interactions with factors I and H. Scand J Immunol. 1986 Mar;23(3):357–363. doi: 10.1111/j.1365-3083.1986.tb01976.x. [DOI] [PubMed] [Google Scholar]
- Nilsson B., Svensson K. E., Borwell P., Nilsson U. R. Production of mouse monoclonal antibodies that detect distinct neoantigenic epitopes on bound C3b and iC3b but not on the corresponding soluble fragments. Mol Immunol. 1987 May;24(5):487–494. doi: 10.1016/0161-5890(87)90023-x. [DOI] [PubMed] [Google Scholar]
- Nilsson L., Nilsson U., Venge P., Johansson O., Tydén H., Aberg T., Nyström S. O. Inflammatory system activation during cardiopulmonary bypass as an indicator of biocompatibility: a randomized comparison of bubble and membrane oxygenators. Scand J Thorac Cardiovasc Surg. 1990;24(1):53–58. doi: 10.3109/14017439009101824. [DOI] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Formation of the initial C3 convertase of the alternative complement pathway. Acquisition of C3b-like activities by spontaneous hydrolysis of the putative thioester in native C3. J Exp Med. 1981 Sep 1;154(3):856–867. doi: 10.1084/jem.154.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. D., Lambris J. D., Cain J. A., Newman S. L. Generation of three different fragments of bound C3 with purified factor I or serum. I. Requirements for factor H vs CR1 cofactor activity. J Immunol. 1982 Nov;129(5):2051–2060. [PubMed] [Google Scholar]
- Salama A., Hugo F., Heinrich D., Höge R., Müller R., Kiefel V., Mueller-Eckhardt C., Bhakdi S. Deposition of terminal C5b-9 complement complexes on erythrocytes and leukocytes during cardiopulmonary bypass. N Engl J Med. 1988 Feb 18;318(7):408–414. doi: 10.1056/NEJM198802183180704. [DOI] [PubMed] [Google Scholar]
- Schreiber R. D., Pangburn M. K., Müller-Eberhard H. J. C3 modified at the thiolester site: acquisition of reactivity with cellular C3b receptors. Biosci Rep. 1981 Nov;1(11):873–880. doi: 10.1007/BF01114821. [DOI] [PubMed] [Google Scholar]
- Sim R. B., Sim E. Autolytic fragmentation of complement components C3 and C4 and its relationship to covalent binding activity. Ann N Y Acad Sci. 1983;421:259–276. doi: 10.1111/j.1749-6632.1983.tb18114.x. [DOI] [PubMed] [Google Scholar]
- Videm V., Fosse E., Mollnes T. E., Garred P., Svennevig J. L. Complement activation with bubble and membrane oxygenators in aortocoronary bypass grafting. Ann Thorac Surg. 1990 Sep;50(3):387–391. doi: 10.1016/0003-4975(90)90480-t. [DOI] [PubMed] [Google Scholar]
- Ward C. A., McCullough D., Fraser W. D. Relation between complement activation and susceptibility to decompression sickness. J Appl Physiol (1985) 1987 Mar;62(3):1160–1166. doi: 10.1152/jappl.1987.62.3.1160. [DOI] [PubMed] [Google Scholar]
- Ward C. A., McCullough D., Yee D., Stanga D., Fraser W. D. Complement activation involvement in decompression sickness of rabbits. Undersea Biomed Res. 1990 Jan;17(1):51–66. [PubMed] [Google Scholar]
- van Oeveren W., Kazatchkine M. D., Descamps-Latscha B., Maillet F., Fischer E., Carpentier A., Wildevuur C. R. Deleterious effects of cardiopulmonary bypass. A prospective study of bubble versus membrane oxygenation. J Thorac Cardiovasc Surg. 1985 Jun;89(6):888–899. [PubMed] [Google Scholar]




